Figure 4.
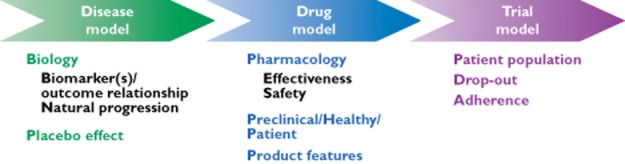
The diagram depicts the major components of a clinical trial simulation (CTS). In model-based drug development, CTS can be used to characterize the interactions between drug and disease, enabling among other things the assessment of disease-modifying effects, dose selection and covariate effects (e.g. age, body weight). In conjunction with a trial model, CTS allows the evaluation of such interactions, taking into account uncertainty and trial design factors, including the implications of different statistical methods for the analysis of the data (Reprinted with permission from Gobburu et al. [39])
