Table 2.
Pharmacokinetic models and parameters describing the time course of drug concentrations in plasma after administration of oral doses of NCE01, NCE02 and NCE03 in conscious dogs (A) and healthy subjects (B)
| A. Dogs | NCE01 | NCE02 | NCE03 |
|---|---|---|---|
| Model structure | – | One-compartment model | – |
| Graphical representation | – |  |
– |
| Parameterization | – | CL*, Vc*, KA | – |
| Interindividual variability | – | CL, Vc, KA | – |
| Covariates | – | Body weight | – |
| B. Healthy subjects | NCE01 | NCE02 | NCE03 |
|---|---|---|---|
| Model structure | – | Dual absorption, two-compartment model | One-compartment model |
| Graphical representation | – | 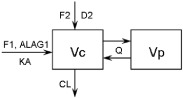 |
 |
| Parameterization | – | CL, Vc, Vp, Q, KA, F1, F2, D2, ALAG1 | CL, Vc, KA, F1 |
| Interindividual variability | – | CL, Vc, Vp, F1, F2 | CL, Vc, KA |
| Covariates | – | Body weight | – |
ALAG, lag time; CL, clearance; D2, duration of zero-order input into compartment 2; F1, bioavailability relative to compartment 1; F2, bioavailability relative to compartment 2; KA, absorption rate constant; Q, intercompartmental clearance; Vc, volume of distribution for the central compartment; Vp, volume of distribution for the peripheral compartment. *CL and V parameters were obtained using individual data fitting in WINNONLIN 4.2.
