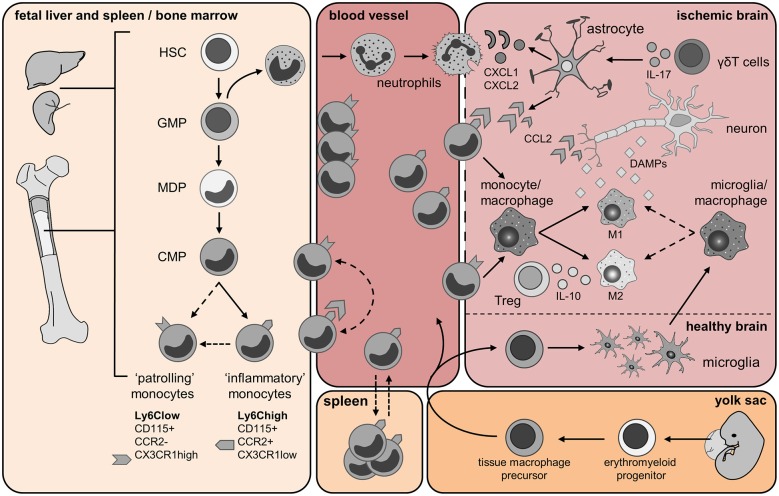
Figure 1.
Origin and trafficking of resident microglia and immune cells of myeloid origin. Fetal liver and spleen/bone marrow panel: Monocytes are generated from hematopoietic stem cells (HSCs) in the fetal liver and during adult life in the bone marrow (BM). The granulocyte macrophage precursors (GMP) give rise to immature neutrophils and the macrophage dendritic cell precursors (MDPs). Monocytes develop from hematopoietic stem cells (HSC) after myeloid lineage commitment through a series of increasingly restricted progenitors (GMP, granulocyte/monocyte progenitor; MDP, monocyte/dendritic cell progenitors). The common monocyte progenitor (CMP) is the direct precursors of mature Ly6Chigh and Ly6Clow monocytes. Ly6Chigh inflammatory monocytes might give rise to circulating Ly6Clow monocytes directly, or via a Ly6Chigh monocyte intermediate. Yolk sac panel: Resident brain microglia have been shown recently to have a different origin than circulating monocytes. During embryonic life, erythroblasts (not shown) and macrophage progenitors are generated in the yolk sac from the common erythro-myeloid progenitor. When the blood circulation is established, macrophage precursors exit the yolk sac and migrate into the developing brain. Embryonic microglia proliferate and are able to renew themselves during gestation and post-natal development as well as in adulthood. Blood vessel and Ischemic brain panels: Few hours after cerebral ischemia onset, mature neutrophils enter the bloodstream upon activation of the CXCR2 receptor and infiltrate the brain in response to chemokines CKLF1 as well as CXCL1 and CXCL2 released by astrocytes. Astrocytic production of these chemokines is dependent upon IL-17 released from brain infiltrating γδT cells. Neutrophils firmly adhere to the endothelium and might either invade the ischemic region or cluster into the perivascular space. During injury, CCL2 is produced by astrocytes, macrophages/microglia and neurons. CCL2 binds its receptor CCR2 expressed by Ly6Chigh inflammatory monocytes, which promotes their egress from the BM into the blood, and then their recruitment from the blood into the injured tissue. Here, these cells give rise to monocyte-derived DC (not shown) and monocyte-derived macrophage populations, which can further polarize into M1 and M2 macrophages. As the ischemic infarct develops, M1 and M2 macrophages contribute to the exacerbation of the damage or wound healing, respectively. Ly6Clow monocytes patrol the blood vessel lumen by associating with the vascular endothelium. Ly6Clow monocytes expressing the CX3CR1 receptor are also recruited to sites of inflammation and possibly contribute to wound healing by differentiating into alternatively activated M2 macrophages. Cerebral ischemia/reperfusion leads to the release of damage-associated molecular pattern (DAMP) molecules from dying neurons. These molecules trigger the activation of resident microglia and astrocytes. Activated microglia promote tissue repair by producing trophic factors and by scavenging necrotic cells. Regulatory T lymphocytes (Treg) secreting IL-10 have shown a protective role in cerebral ischemia and might promote macrophage M2 polarization. Spleen panel: An interesting twist to the origin of recruited monocytes during injury has been added by the recent identification of a major monocyte reservoir in the spleen of mice. Following ischemic myocardial injury, splenic monocytes are mobilized to the site of inflammation and participate in tissue injury (Swirski et al., 2009). Dashed arrows represent findings that are not clearly defined yet and need further investigations in context of cerebral ischemia.