FIG 4.
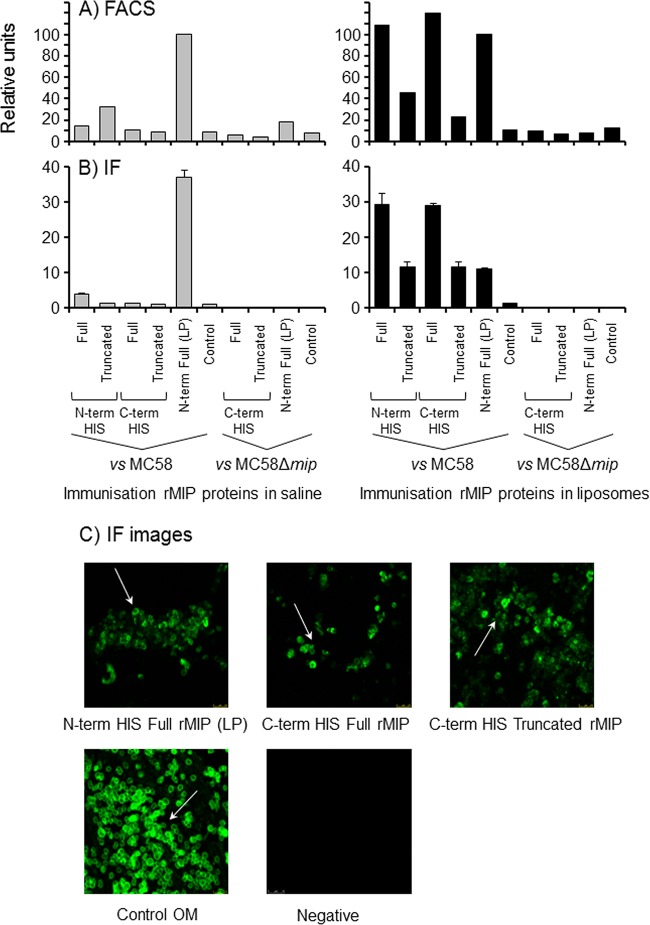
Antibodies to rMIP proteins recognize MIP on the surface of meningococci. (A) FACS analysis demonstrates anti-rMIP antibody binding to the surface of meningococci. Pooled murine antisera to rMIP constructs in either saline solution or liposomes were reacted (1/10) with fixed whole MC58 cells and the Δmip mutant. The column data are presented as a percentage of the reactivity of the N-term His full rMIP (LP) protein, which has been shown previously to induce bactericidal antibodies and to bind to meningococcal cells (22). Data are representative of the results of experiments done with the pooled samples (n ≥ 2 experiments). (B) Immunofluorescence (IF) staining confirms binding of anti-rMIP antibodies to the surface of meningococci. Pooled murine antisera to rMIP constructs were reacted (1/50) with fixed whole MC58 cells and the Δmip mutant. IF staining was quantified as described in Materials and Methods. The columns represent the mean ROI values, and the error bars represent the standard errors of the means (of 3 independent areas of staining measured in triplicate). The data are from a representative experiment (n ≥ 3 experiments for pooled samples). (C) Representative confocal images of immunofluorescence staining of MC58 meningococcal surfaces with antisera raised to N-term His full rMIP (LP), C-term His full rMIP, and C-term truncated rMIP, delivered in liposomes. Similar staining patterns were observed with antisera raised to N-term His full and truncated rMIP proteins in liposomes. The positive control was antisera raised to whole MC58 OM, and a representative negative-control (sham immunization) image is shown. Images were captured at ×60 magnification.