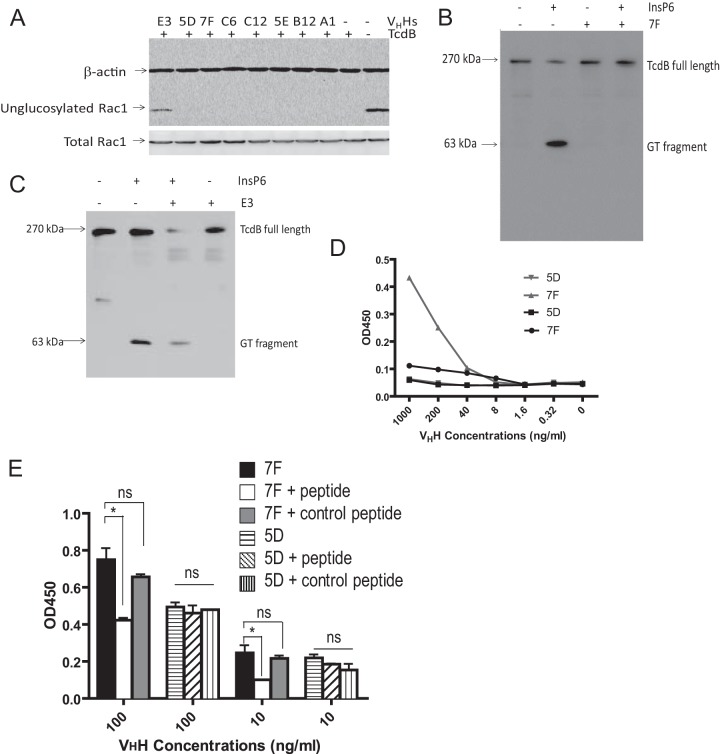
FIG 2.
E3 and 7F inhibit glucosyltransferase and autoprocessing activities, respectively. (A) E3, but not 7F or other tested VHHs, affects the glucosyltransferase activity of TcdB. Vero cell lysates were analyzed for Rac1 glucosylation by immunoblotting after exposure to wild-type TcdB and/or E3 or by 7F VHHs. A monoclonal antibody (clone 102) that binds only to nonglucosylated Rac1 was used as a probe. β-Actin was used as an equal loading control. (B) 7F blocks the autocatalytic cleavage of GTD from full-length TcdB. TcdB was incubated in the presence or absence of 7F and InsP6. The autocleavage and release of GTD from full-length TcdB were assessed by immunoblotting using a monoclonal antibody against GTD. (C) E3 does not block the autocleavage of GTD from full-length TcdB. (D) The peptide consisting of aa 520 to 543 binds specifically to 7F. Ninety-six-well plates were coated with the peptide consisting of aa 520 to 543 or aa 422 to 439 and incubated with serial dilutions (0.32 to 1,000 ng/ml) of 7F or control VHH 5D. Goat anti-E tag-IgG-HRP was used as a secondary antibody. (E) The peptide consisting of aa 520 to 543 significantly reduces binding of 7F to GTD. Ninety-six-well plates were coated with 0.5 μg/ml TcdB and incubated with 7F or 5D VHH alone (100 or 10 ng/ml) or with the VHHs plus the peptide consisting of aa 520 to 543 or aa 422 to 439. Goat anti-E tag-IgG HRP was used as a secondary antibody. *, P < 0.05; ns, not significant.