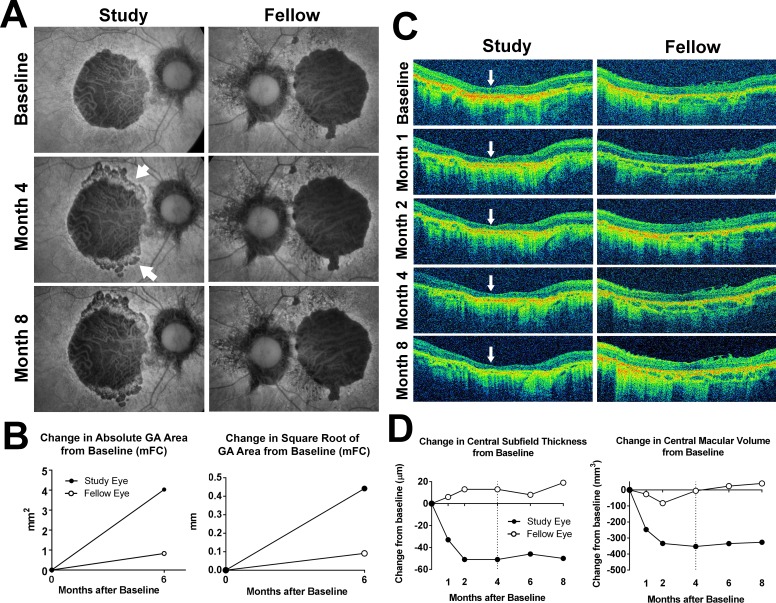
Figure 1.
Fundus changes in participant 6 during study treatment and follow-up. (A) Fundus autofluorescence images were obtained using a modified fundus camera (mFC). The right eye was randomized to receive investigational product (Study) while the left (Fellow) eye was observed without treatment. At month 4, after receiving two intravitreal injections of investigational product, the central GA lesion in the study eye demonstrated an expansion in the area of hypoautofluorescence at the superior and inferior margins (arrowheads). Investigational product administration was suspended at this month 4 visit and withheld thereafter. At month 8, the size and appearance of the GA lesion in the study eye appeared relatively stable. The fellow eye demonstrated no marked changes. (B) Changes in absolute (left) and square root transform (right) of GA area in the study and fellow eye at month 6 from baseline were measured from mFC FAF images. (C) Horizontal B-scans aligned through the center of the macula for the study and fellow eyes from baseline to month 8 were obtained. Marked central retinal thinning was observed at month 1 (indicated by arrows) in the study eye but not in the fellow eye. (D) Change in central retinal thickness (left) and central macular volume (right) from baseline to month 8 documented a rapid decline in the first 2 months in the study eye, which stabilized following drug suspension at month 4 (dashed line).