Figure 2. trT2-50 binds actin.
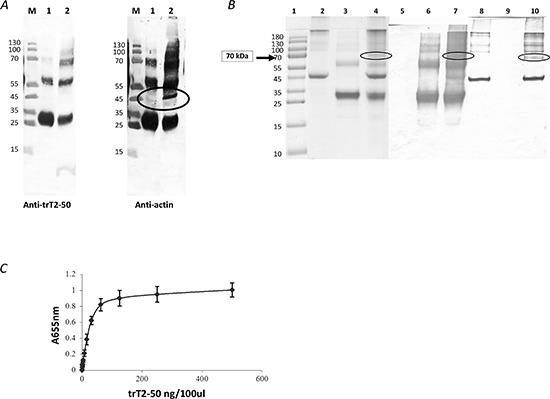
trT2-50 binds cell surface actin (A). M, molecular markers. Lane 1, eluted fraction: his tag-trT2-50 was immobilized to Nickel beads. Supernatant solution of heparan-treated confluent starved HUVEC was added and incubated in rotation. Bounded fractions were eluted using 0.5M imidazol. Lane 2, supernatant solution of heparan-treated confluent starved HUVEC incubated with trT2-50 (without further purification). trT2-50 binds actin in vitro (B). trT2-50 and actin form a complex of 70kDa in solution. Actin (10 μg) was incubated with 20 μg trT2-50 in Buffer G for 30 min at room temperature. EDC was then added and incubated for another 30 min. The reaction was then terminated and the cross-linked complex was analyzed by 10% SDS-PAGE stained with Coomassie blue or further analyzed by Western blot analysis upon incubation with anti-trT2-50 or anti-actin antibodies. Lane 1, molecular markers. Lanes 2-4, Coomassie blue staining: actin, trT2-50 and complex formation, respectively. Lanes 5-7, Western blot analysis using anti-trT2-50: actin, trT2-50 and complex formation, respectively. Lanes 8-10, Western blot analysis using anti- actin: trT2-50, actin and complex formation, respectively. trT2-50 binds actin on solid phase (ELISA) (C). trT2-50 was added to immobilized actin at increasing concentrations from 0 to 500 ng/100μl/well, and then reacted with rabbit anti-trT2-50 and goat anti rabbit IgG-HRP. The amount of bound trT2-50 was quantified by measuring the optical density at 655 nm.
