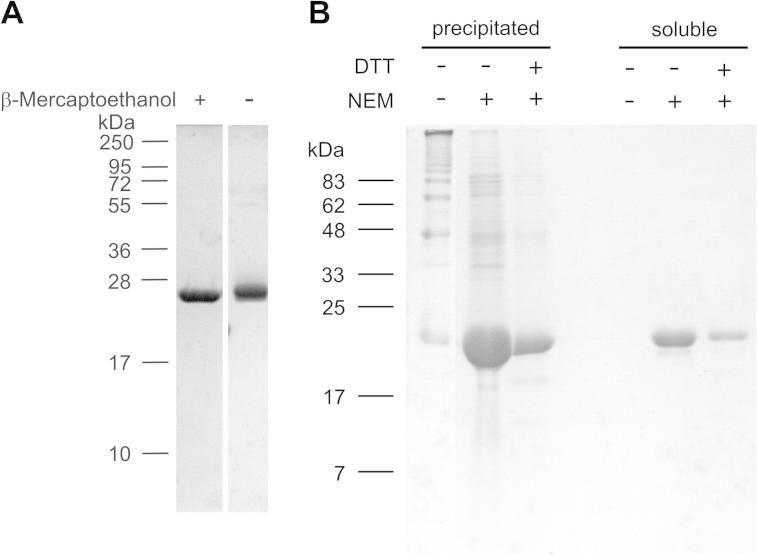
Fig. 2.
Analysis of disulfide bonding of the matrilin-4 VWA2 domain expressed in E. coli. (A) The matrilin-4 VWA2 domain was subjected to electrophoresis on a 15% (w/v) SDS–polyacrylamide gel with and without prior reduction with β-mercaptoethanol. (B) Matrilin-4 VWA2 was purified without buffer additives and in the presence of 1 mM NEM or 1 mM NEM and 10 mM DTT, respectively. After visible protein precipitation in the elution fractions, precipitated and remaining soluble protein were subjected to SDS–PAGE without reduction on a 15% polyacrylamide gel and stained with Coomassie. Left: molecular mass marker in kDa.