FIGURE 6.
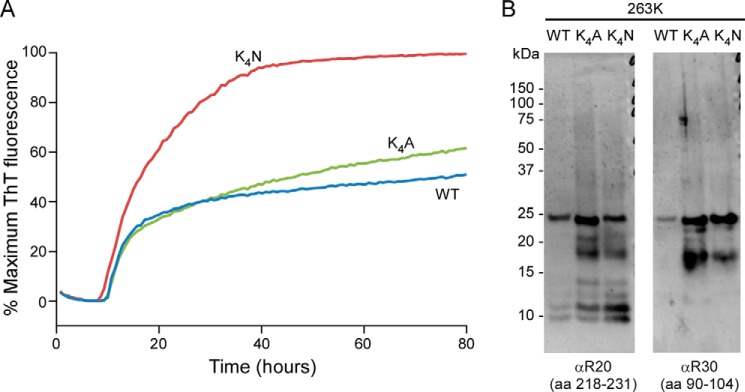
Prion-templated amyloid formation under neutral, nonchaotropic RT-QuIC conditions. A, 263K scrapie-infected brain homogenate was used to seed fibril formation by WT, K4A, and K4N rPrP 23–231 substrates in RT-QuIC reactions. The data points represent average percentages of maximum ThT fluorescence from four replicate wells as a function of reaction time. Similar results were obtained in at least six independent experiments. B, Western blot analysis of scrapie-seeded, PK-treated RT-QuIC products using antisera to residues 90–104 or 218–231 (R30 and R20, respectively). Extended 17-kDa PK-resistant core fragments reactive with both antisera were always more prominent in the K4A and K4N fibrils than in the WT fibrils in at least six experiments.
