FIGURE 3.
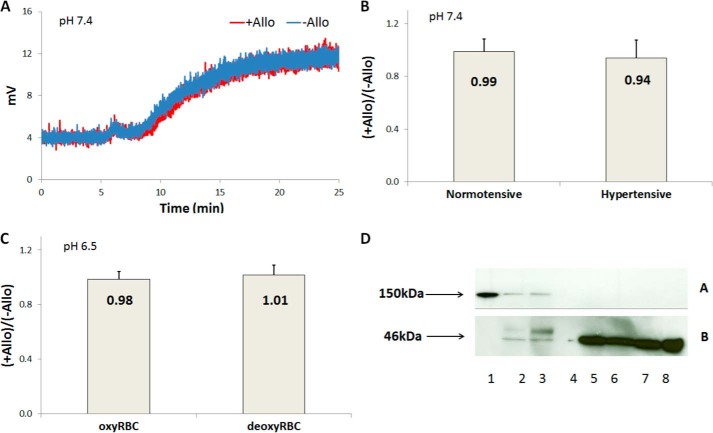
Further studies examining the role of XOR in nitrite reductase activity of XOR using chemiluminescent detection of NO at 37 °C. A, representative signals of chemiluminescence due to NO production from nitrite reduction by adding deoxygenated RBCs to a deoxygenated nitrite solution using RBCs from normotensive volunteers in the presence (+Allo in red) or absence of allopurinol (−Allo in blue) at pH 7.4. Deoxygenated RBCs (0.5 Hct) were added to a nitrite solution (20 ml, 20 mm) after ∼5 min after recording the baseline signal, and NO production was observed for 20 min. B, the ratio of NO production by deoxygenated RBCs (n = 5) in the presence or absence of allopurinol at pH 7.4. No significant difference was found in the presence or absence of allopurinol using RBCs from normotensive (p = 0.7) or hypertensive (p = 0.2) volunteers. C, the ratio of NO production using RBCs at pH 6.5 (n = 3). No significant difference was found between NO production in the presence or absence of allopurinol. The ratio of NO production in the presence or absence of allopurinol from nitrite reduction by adding oxygenated RBCs to a deoxygenated nitrite solution (20 ml, 20 mm) at pH 6.5 was 0.98 (n = 3) and by adding deoxygenated RBCs to a deoxygenated nitrite solution (20 ml, 20 mm) was 1.01 (n = 3). No significant difference was found in the presence or absence of allopurinol for oxygenated (p = 0.8) or deoxygenated (p = 0.8) RBCs. D, Western blot for erythrocytic XOR. Panel A is an immunoblot for XOR, and panel B is an immunoblot for β actin. Lanes were loaded as follows: bovine-purified XOR (lane 1), mouse liver lysate (lane 2), human liver lysate (lane 3), empty (lane 4), human RBC ghost lysates (lanes 5–8). The purified bovine XOR, mouse, and human liver lysates show protein with a molecular mass consistent with the monomer of XOR at 150 kDa, whereas RBC ghost preparations do not.