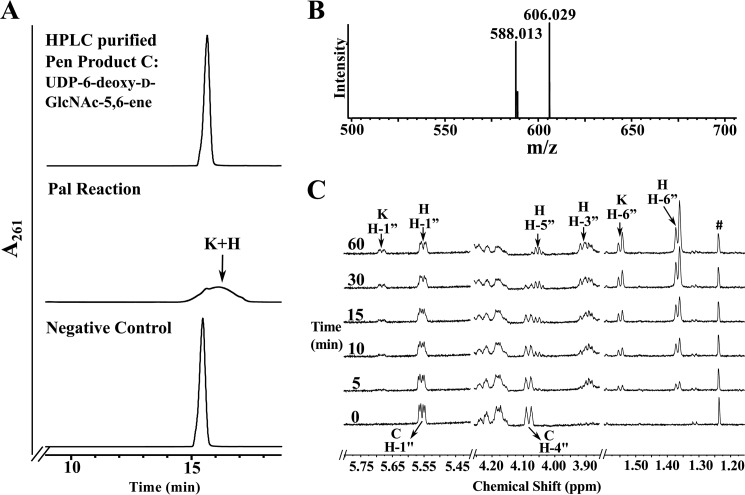
FIGURE 6.
Analyses of UDP-4-keto-6-deoxy-l-AltNAc, the enzymatic product of Pal by UV-HPLC, mass spectrometry, and time-resolved 1H NMR. A, purified Pal was reacted with UDP-6-deoxy-d-GlcNAc-5,6-ene (“C,” the purified enzymatic product of Pen) to yield at least two new UV-broad HPLC peaks labeled “K,” “H,” and marked by arrows. The top HPLC trace in A corresponds to purified product C standard, and the bottom trace is the negative control. an assay of purified irrelevant protein with C. The K and H peaks (eluted from HILIC column at 16 min) were collected and characterized by NMR (see Table 2). B, MS analysis in negative mode gives two [M − H]− ions at m/z 588.1 and 606.0 corresponding to two forms of UDP-4-keto-6-deoxy-l-AltNAc, 4-keto and its hydrated forms, respectively. C, time-resolved 1H NMR showing Pal enzymatic conversion of UDP-6-deoxy-d-GlcNAc-5,6-ene (C) to UDP-4-keto-6-deoxy-l-AltNAc. As the reaction proceeds, two molecular species are produced, a 4-keto form and a 4-keto-hydrated form of UDP-4-keto-6-deoxy-l-AltNAc. The selected chemical shift region of the proton NMR spectrum that corresponds to the H-1″ anomeric proton of enzymatic reactant and product is shown between 5.80 and 5.40 ppm. Note the H-4″ peak of the substrate (“C”) shown at 4.09 ppm is converted to new product K and H (the 4-keto and its hydrated form). The far right panel (between 1.60 and 1.30 ppm) shows the methyl groups (H-6″) belonging to the hydrated (H) and the keto (K) forms of the product UDP-4-keto-6-deoxy-l-AltNAc.