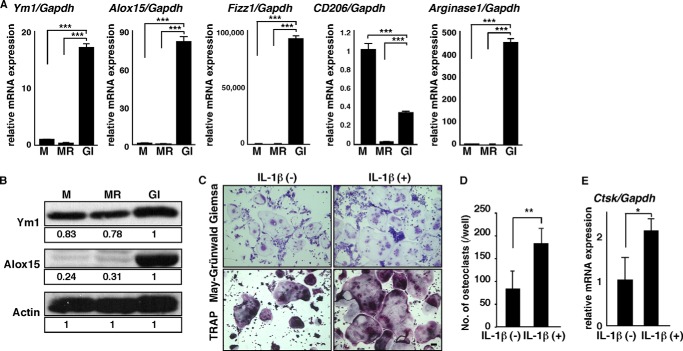
FIGURE 3.
FBGCs express wound-healing molecules, and IL-1β stimulates osteoclastogenesis. A and B, osteoclast and FBGC common progenitor cells were cultured in the presence of M-CSF alone (M) to induce macrophages, M-CSF plus RANKL (MR) to induce osteoclasts, or GM-CSF plus IL-4 (GI) to induce FBGCs. Expression of Ym1, Alox15, Fizz1, CD206, or ariginase1 transcripts relative to Gapdh and expression of Ym1 and Alox15 protein were analyzed by quantitative real time PCR (A) and Western blotting (B), respectively. Data represent means ± S.D. of Ym1/Gapdh, Alox15/Gapdh, Fizz1/Gapdh, CD206/Gapdh, or arginase1/Gapdh levels (***, p < 0.001; n = 3). Actin protein expression served as an internal control. Relative Ym1 or Alox15 protein levels determined by immunoblot were quantified by densitometry and are shown as values relative to levels in FBGCs (GI). Representative data of at least three independent experiments are shown. C and D, osteoclast and FBGC common progenitor cells were cultured in the presence of M-CSF plus RANKL with or without IL-1β (10 ng/ml) for 5 days and then subjected to May-Grünwald Giemsa and TRAP staining (bar, 100 μm) (C). The number of multinuclear osteoclasts containing more than three nuclei was then scored (**, p < 0.01, n = 3) (D). E, total RNAs were prepared from osteoclasts treated with or without IL-1β (10 ng/ml) for 3 days, and Ctsk expression relative to Gapdh was analyzed by quantitative real time PCR. Data represent means ± S.D, of Ctsk/Gapdh levels (*, p < 0.05; n = 3). Representative data from at least three independent experiments are shown.