FIGURE 8.
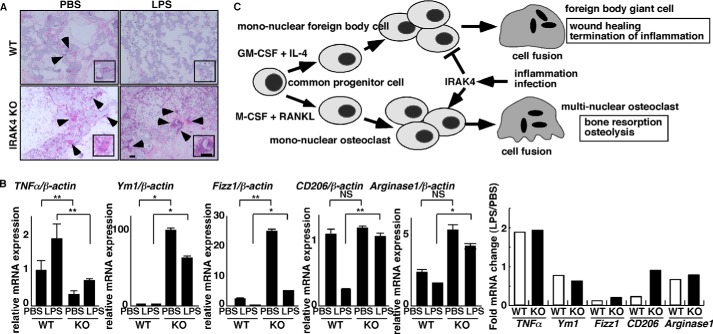
IRAK4 is a potential therapeutic target regulating the FBR in vivo. A and B, foreign bodies (PVA sponges soaked with PBS or LPS (25 mg/kg)) were implanted into peritoneal spaces in wild-type or IRAK4-deficient mice. After 6 days, foreign bodies were dissected, and tissue sections were stained with H&E (A) (bar, 100 μm). Alternatively, total RNAs were prepared from cells contained in foreign bodies and TNFα, Ym1, Fizz1, CD206; and arginase1 expression relative to β-actin was analyzed by quantitative real time PCR (B, left panels). Data represent means ± S.D. of TNFα/β-actin, Ym1/β-actin, Fizz1/β-actin, CD206/β-actin, or arginase1/β-actin levels (*, p < 0.05; **, p < 0.01; NS, not significant; n = 5–12) (B, right panel). Fold changes in mRNA expression between LPS-induced versus PBS-treated samples, shown as LPS/PBS. Representative data of at least three independent experiments are shown. C, schematic model of FBGC and osteoclast formation regulated by IRAK4 under inflammatory or infectious conditions. FBGC or osteoclast formation is induced in the presence of GM-CSF plus IL-4 or M-CSF plus RANKL, respectively, from common progenitor cells. Both FBGCs and osteoclasts form multinuclear cells by fusion of mononuclear cells. Inflammation or infection activates IRAK4 and inhibits FBGC formation while stimulating osteoclastogenesis, an event underlying implant failure. Multinucleation of FBGCs likely elevates wound healing efficiency, although osteoclasts mediate bone-resorbing activity.