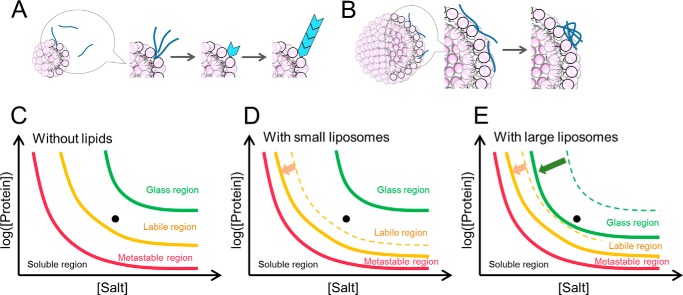
FIGURE 8.
Schematic models of amyloid nucleation and amorphous aggregation on the surface of liposome membranes. A and B, schematic mechanism of the Aβ-(1–40) fibrillation in the presence of liposomes. In the presence of smaller liposomes, Aβ-(1–40) interacts with the surface of membrane weakly, and this weak interaction promotes the nucleation. Aβ-(1–40) is easy to remove due to this interaction and the elongation reaction is similar to without lipids (A). In the presence of larger liposomes, Aβ-(1–40) interacts with the surface of membrane more strongly than that of smaller liposomes. This stronger interaction makes it difficult to induce nucleation and leads some Aβ peptides to amorphous aggregates. Other Aβ peptides exist in medium, and they form amyloid fibrils or amorphous aggregates (B). C–E, schematic phase diagram of conformational states of Aβ-(1–40) in the absence (C) or presence of small (D) or large vesicles (E). The locations of the protein solutions as studied here are indicated by dots.