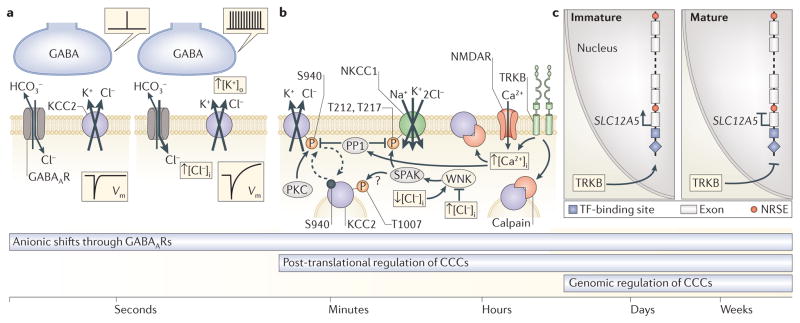
Figure 2. Mechanisms of ionic plasticity and their temporal domains.
Cation-chloride cotransporters (CCCs) control ionic plasticity across various overlapping time scales that range from seconds to weeks, and beyond. a | Short-term ionic plasticity of GABAergic signalling is based on fast, activity-dependent transmembrane movements of Cl− and HCO3−, which alter the driving force and polarity of GABA-induced currents and thereby cause changes in the postsynaptic membrane potential (Vm). On the left, a single presynaptic event leads to a hyperpolarization of Vm. On the right, repetitive activation of GABAergic terminals evokes a biphasic Vm response. In biphasic Vm responses, the depolarizing HCO3− current leads to an increase in GABAA receptor (GABAAR)-mediated uptake of Cl− and an increase in intracellular Cl− concentration ([Cl−]i). The consequent K+–Cl− cotransporter 2 (KCC2)-mediated extrusion of K+ increases the extracellular K+ concentration ([K+]o) and has a depolarizing or even functionally excitatory action on Vm. b | Fast functional regulation of CCCs on a time scale of minutes to hours is mediated by post-translational mechanisms, including (de)phosphorylation of key residues on the intracellular domains of KCC2 and Na+–K+–2Cl− cotransporter 1 (NKCC1) (see also FIG. 1d) and calpain-mediated cleavage of KCC2. Constitutive membrane recycling (dashed arrows) of KCC2 is regulated by the phosphorylation state of the C-terminal serine residue S940. Phosphorylation of S940 by protein kinase C (PKC) limits clathrin-mediated endocytosis of KCC2. By contrast, protein phosphatase 1 (PP1)-dependent dephosphorylation of S940 leads to internalization of KCC2 and a reduction in neuronal Cl− extrusion capacity following intense activation of NMDA receptors (NMDARs) and an increase in [Ca2+]. Under such conditions, KCC2 is also C-terminally cleaved by the Ca2+- and brain-derived neurotrophic factor (BDNF)-activated protease calpain, which results in irreversible inactivation of KCC2 (BOX 3). NKCC1 is kinetically regulated through a Cl−-sensing cascade involving WNK (with no lysine kinase) and the STE20-related kinases SPAK and OSR1 (oxidative stress responsive kinase 1; not shown). WNKs are allosterically modulated by Cl−, with a decrease in [Cl−]i leading to activation of SPAK and OSR1 by WNKs. Consequent SPAK-mediated phosphorylation of key N-terminal threonine residues (for example, T212 and T217; see also FIG. 1d) of NKCC1 results in its activation and Cl− accumulation. By contrast, dephosphorylation of these residues by PP1 inactivates NKCC1. Reciprocal regulation of transport activities of NKCC1 and KCC2 by the WNK SPAK and WNK OSR1 cascade has been demonstrated in heterologous expression systems and may also take place in neurons. At least one SPAK and OSR1 phosphorylation site (T1007) is found in KCC2b, the principal KCC2 splice variant found in neurons. However, it is not clear how phosphorylation of KCC2 by SPAK and OSR1 affects neuronal Cl− extrusion capacity. c | Neuron-specific expression of KCC2 is ensured via multiple transcriptional mechanisms, including the actions of neuron-restrictive elements (NRSEs; also known as RE1), which silence SLC12A5 (encoding KCC2) in non-neuronal cells and neuron-enriched transcription factors (TFs). Neuron-enriched TFs, for example, members of the early growth response (EGR; not shown) family, are sensitive to signalling by neurotrophic factors, such as BDNF and its receptor tropomyosin-related kinase B (TRKB), which exert qualitatively different effects on SLC12A5 transcript expression in immature and mature neurons. The TRKB-mediated cascades may reverse during neuronal trauma, resulting in recapitulation of immature-like Cl− homeostasis in diseased neurons (BOX 3). Long-term consolidation of changes in KCC2 following trauma or during epileptogenesis is likely to be mediated to an extent by the above transcriptional mechanisms.