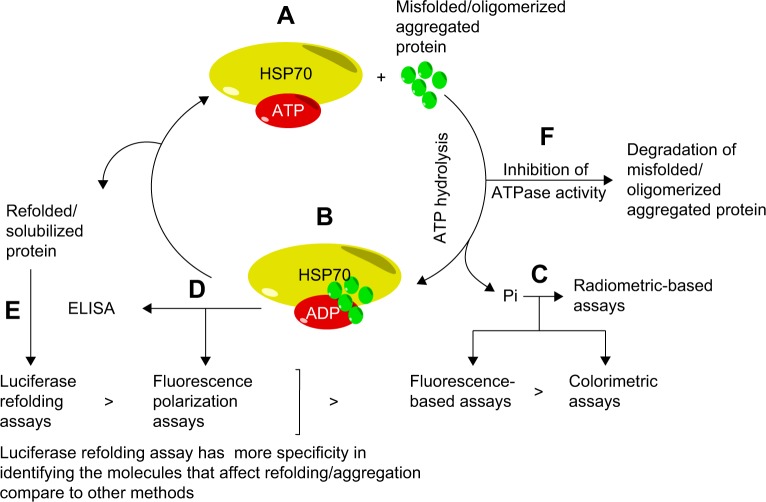
Figure 1.
Methods for measurement of HSP70 ATPase activity. Inhibition and activation of HSP70 ATPase activity can be estimated with the following methods.
Notes: HSP70 binding to ATP (A) has less affinity for misfolded/aggregated/oligomerized protein than when the ATP is hydrolyzed to ADP. Once the phosphate is released, HSP70 can bind to misfolded protein (B). ATP hydrolysis, through measurement of released phosphate is determined by different assays (C). HSP70, in the ADP-bound conformation, can be detected by a different set of assays (D). Released refolded protein is detected by the luciferase refolding assay (E). Inhibition of HSP70 ATPase activity inhibits refolding/antiaggregation or antioligomerization and can activate degradation pathways, such as ubiquitin pathways, to eliminate the aggregated proteins (F). Pi: Phosphate. >: Specificity and selectivity. “>” greater than symbol compares specificity and selectivity of assays in identifying molecules that affect refolding/aggregation.
Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; ATPase, adenosine triphosphatase; HSP70, heat shock protein 70; ELISA, enzyme-linked immunosorbent assay; Pi, phosphate.