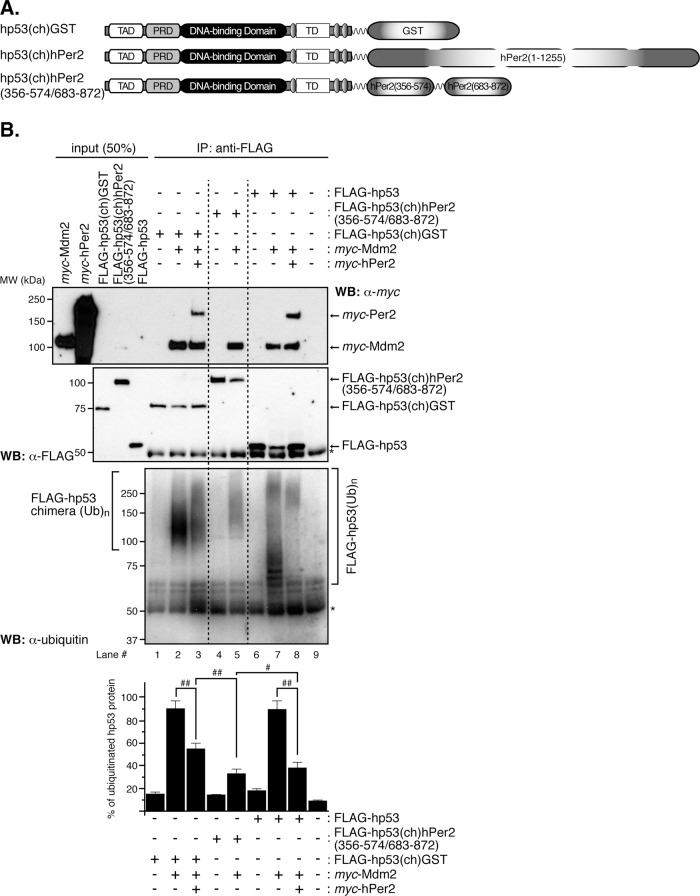
FIGURE 2:
In vitro ubiquitination of hp53 is compromised when stably bound to hPer2. (A) Schematic representation of all chimeras designed for this study. Constructs were 5′ FLAG-tagged, myc-tagged, or untagged upstream of hp53, followed by downstream cloning of GST, hPer2, or hPer2(356-574/683-872) open reading frames. A linker encoding for six Gly was inserted between both the hp53 and hPer2 genes and the two hPer2-coding fragments. (B) In vitro transcribed and translated FLAG-hp53, FLAG-hp53(ch)hPer2(356-574/683-872), FLAG-hp53(ch)GST, myc-Mdm2, and myc-hPer2 proteins were used for ubiquitination experiments. When indicated, myc-hPer2 and either FLAG-hp53 or FLAG-hp53(ch)GST were preincubated; thus the complex was formed before adding myc-Mdm2. For the FLAG-hp53(ch)hPer2(356-574/683-872) chimera, the translated protein and myc-Mdm2 were incubated together before the ubiquitination reaction took place. Ubiquitination was carried out as described in Materials and Methods. FLAG-tagged proteins were immunoprecipitated with α-FLAG/protein A beads and blotted using α-ubiquitin antibody. Membranes were then stripped and reprobed with α-FLAG and -myc antibodies to detect complex bound proteins. Asterisk indicates IgG heavy chain. Immunoblot data from a single experiment repeated three times with similar results. Quantification of the sample's ubiquitinated signal was performed using ImageJ, version 1.45 (bar graph). Statistical comparisons were evaluated by t test. #p ≤ 0.05; ##p < 0.005.