Fig. 5.
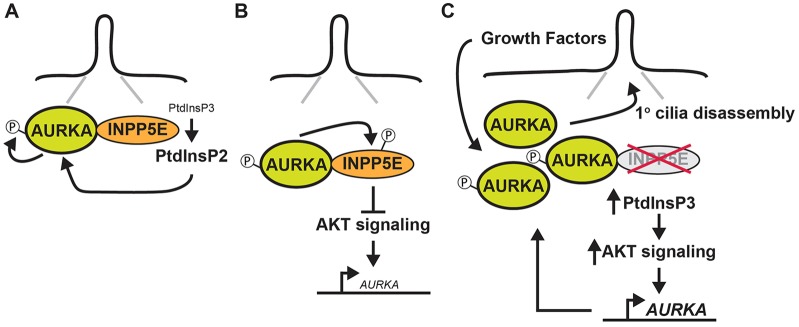
A model for reciprocal interactions between INPP5E and AURKA. (A) At the primary cilium we propose that hydrolysis of PtdIns(3,4,5)P3 by INPP5E alters the levels of different phosphoinositide species and, in so doing, mediates the functional interaction between INPP5E and AURKA, resulting in the activation of the latter protein through auto-phosphorylation. (B) Activated AURKA binds to and phosphorylates INPP5E, increasing its phosphatase activity and reducing AURKA transcription (small font) downstream of AKT. (C) When INPP5E is deleted or mutated, increased AKT signaling elevates AURKA transcription (large font), resulting in a generalized accumulation of AURKA protein at the cilia. In response to physiological stimuli, we propose that the increased pool of AURKA at this site drives the abnormal cilia disassembly phenotype observed in cells lacking INPP5E.
