Abstract
A continuous leukocyte cell line with phagocytic activity was established from peritoneal macrophages of rohu, Labeo rohita (LRPM). LRPM was initiated from adherent mononuclear leukocytes isolated from peritoneal cavity of rohu, without use of any growth factors or feeder cells. These cells exhibited maximum growth at 30 °C in L-15 medium containing 20 % foetal bovine serum, and has been subcultured for more than 60 passages till date. The cells showed 85 % viability after 6 months of storage in liquid nitrogen. The species of origin of the LRPM was confirmed by the amplification and sequencing of 655 bp fragment of cytochrome oxidase subunit I of mitochondrial DNA. Functionally, LRPM showed phagocytic activity of yeast cells and fluorescent latex beads as evaluated by phase contrast and scanning electron microscopy, respectively. Immuno-modulators such as bacterial lipopolysaccharide and phorbol myristate acetate resulted in functional activation of LRPM; and enhanced their microbicidal activity through release of reactive oxygen species and nitric oxide. Culture supernatant from activated cells also revealed lysozyme activity. Cells of LRPM were positive for alpha-naphthyl acetate esterase enzyme indicating macrophage lineage. Our results indicate that this cell line can be a useful in vitro tool to study the role of macrophages in teleost immune system and to evaluate the effects of new aquaculture drugs. The LRPM cell line represents the first reported leukocyte cell line of peritoneal origin from any freshwater species of fish.
Keywords: Labeo rohita, Cell line, Macrophage, Peritoneal cavity, Phagocytosis
Introduction
Teleost fish immune system includes most of the elements of the innate immune system present in mammals (Sunyer 2013). In teleosts, innate immunity plays a decisive role in defense against invading pathogenic organisms. Therefore, cells of the monocytic/macrophage lineage have drawn significant interest in studies involving mechanisms of host–pathogen interactions in fish (Russo et al. 2009). Macrophages in fish are the principal phagocytic cells, which phagocytose inert or antigenic materials, exert cytotoxic activity and stimulate lymphocyte proliferation by secreting interleukin-1-like factors (Secombes and Fletcher 1992). Macrophages play an important role in specific as well as non-specific immune responses by means of their antigen-presenting function, secretion of cytokines and production of non-specific humoral defense components (McCarthy et al. 2008). Assays of macrophage function have been used as indicators of fish health (Secombes and Fletcher 1992). Fish macrophages are usually obtained from the kidney, spleen and the peritoneal cavity. The peritoneal cavity is a unique compartment within which a variety of immune cells reside, and from which macrophages are commonly drawn for functional studies (Ghosn et al. 2010). Macrophages from fish are difficult to purify due to heterogeneous cell populations of different maturity (Jenkins and Klesius 1998). Peritoneal cavity is a membrane-bound cavity which contains the liver, spleen, most of the gastro-intestinal tract and other viscera. It harbors a number of immune cells including macrophages, B cells and T cells. The presence of a high number of macrophages in the peritoneal cavity makes it a preferred site for the collection of resident macrophages (Zhang et al. 2008). Macrophages have been characterized from several fish species and it is widely acknowledged that they share many similarities from one fish species to another. Leukocyte cell line provides useful tool to assess immunological defense mechanisms and immune status of fish (Datta et al. 2009). Many fish macrophage/monocyte cell lines are reported from peripheral blood leukocytes (Vallejo et al. 1991; Weyts et al. 1997; Dewitte-Orr et al. 2006; Chaudhary et al. 2012a), head kidney leukocytes (Wang et al. 1995) and thymus (Chaudhary et al. 2012b).
In the present study, we report the establishment and characterization of a leukocyte cell line from peritoneal cavity of rohu, Labeo rohita (LRPM), an important food fish of India. This leukocyte cell line was characterized morphologically by light and electron microscopy. LRPM cell line exhibits important functional properties such as phagocytosis and production of reactive oxygen species (ROS), nitric oxide and lysozyme activity on appropriate stimulation.
Materials and methods
Fish
Healthy L. rohita (300–500 g) obtained locally were maintained in fibre reinforced plastic tanks and provided pelleted fish feed every day. Fish were acclimated to the laboratory conditions for 15 days prior to experiment.
Isolation and culture of peritoneal macrophages
Peritoneal macrophages were isolated as per the method described by Ishibe et al. (2008), with some modifications. Briefly, rohu were euthanized with MS-222 (Sigma-Aldrich, St. Louis, MO, USA) and 5 mL PBS containing 40 units/mL of heparin was then injected through the peritoneal wall at the midline using 5 mL syringe attached with a 22-G needle. Using the same syringe system, peritoneal fluid was gently withdrawn from the specimen. This procedure was repeated twice and the harvested cell suspensions were pooled in siliconized centrifuge tubes on ice. The cells were washed with PBS by centrifugation (200×g, 10 min at 24 °C). The final cell pellet was resuspended in a 4 mL PBS, layered over 4 mL Histopaque (Sigma-Aldrich) and centrifuged at 400×g for 30 min at 24 °C without using the brakes. Mononuclear cells (MNCs) were collected from interface, diluted with PBS and centrifuged at 250×g for 10 min. Cultures were initiated by seeding the MNC’s in a 25 cm2 culture flasks (Nunc, Roskilde, Denmark) containing 25 mL of L-15 medium (Gibco BRL, Gaithersburg, MD, USA) supplemented with 20 % foetal bovine serum (FBS, Sigma-Aldrich) and antibiotic–antimycotic solution (Invitrogen, Carlsbad, USA). After 24 h of incubation at 30 °C, the non-adherent cells were removed by washing the cultures three times with PBS. Residual non-adherent cells were removed by washing the cultures again after 2–3 days. After formation of monolayer, the cells were subcultured at regular intervals using trypsin–EDTA (Invitrogen, Bangalore, India).
Effect of temperature and FBS on cell growth
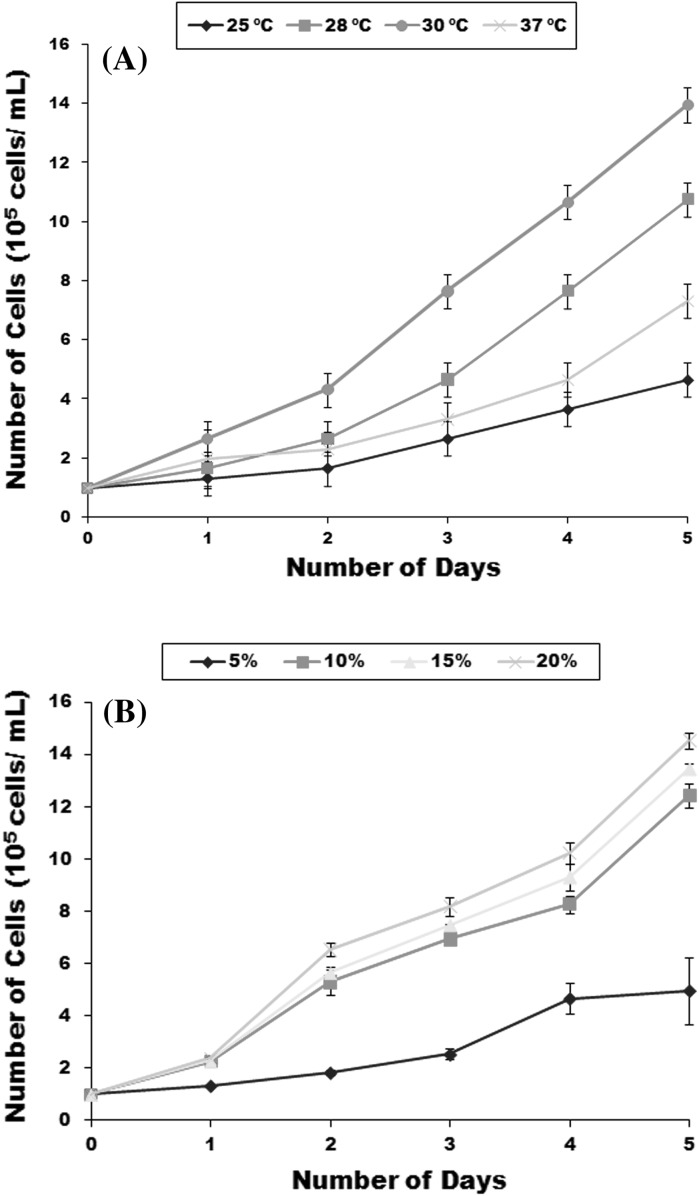
The effect of temperature and FBS concentration on growth of LRPM was studied at the 30th passage level. Cells at a concentration of 1 × 105/mL were inoculated in cell-culture flasks and incubated at 30 °C for 2 h to allow attachment. Then batches of flasks were incubated at selected temperatures of 25, 28, 30, and 37 °C for growth tests. Every day, duplicate flasks at each temperature were washed with PBS twice after which 0.2 mL of 0.25 % trypsin solution (Invitrogen, Carlsbad, CA, USA) was added to each flask. When the cell rounded up, the cell density was measured by a haemocytometer and the numbers were expressed as cells per mm3. The experiment was carried out for 5 days in triplicate. The growth response to different concentrations of FBS (5, 10, 15 and 20 %) on cell growth was assessed in triplicate 25 cm2 flasks using the same procedure as mentioned above at 30 °C.
Cryopreservation of cultured macrophages and revival of frozen cells
Forty-eight hour-old cultures at the 30th and 60th passages were harvested by centrifugation and suspended in L-15 medium containing 10 % FBS and 10 % dimethyl sulphoxide at a density of 105 cells/mL. The cell suspensions were dispensed into 2 mL plastic ampoules and kept initially at −20 °C for 4 h and then at −80 °C overnight and finally transferred into liquid nitrogen (−196 °C). The frozen cells were recovered from storage after 6 month post-storage by thawing quickly with running water at 30 °C. Following removal of the freezing medium by centrifugation, the cells were suspended in L-15 with 10 % FBS and tested for viability by haemocytometer counting after trypan blue staining. The cells were seeded into a 25 cm2 cell culture flask and observed for growth. All experiments were done in triplicate.
Phagocytosis
Demonstration by light microscopy
The phagocytic activity of the cultured peritoneal macrophages was tested with baker’s yeast (Saccharomyces cerevisiae), by the following procedure. The yeast cell suspension was prepared following Roy and Rai (2004). Briefly, the yeast cells were heat-killed at 80 °C for 15 min and washed twice with PBS. The pellet was finally suspended in culture medium supplemented with 5 % FBS to get a concentration of approximately 106 cells/mL. LRPM (1 × 105 cells) were seeded in sterile coverslips kept in a sterile petriplates and allowed to grow for 24 h in L-15 medium. Next day, 100 μl of yeast suspension was then placed over the cover slips. The plate was then incubated for 90 min in a humid chamber at 30 °C. After the incubation period, the coverslips were washed twice in PBS, followed by fixing with 100 % methanol. Cells were stained with Giemsa (Merck, Mumbai, India) and observed under phase contrast microscope (Norum et al. 2005). As a control, cells from Epithelioma papulosum cyprini (EPC) cell line were also seeded on coverslips separately and similar procedure was followed as described above.
In another experiment, fluorescent latex beads, amine-modified polystyrene (2 μm diameter, Sigma) suspended in L-15 were then placed over LRPM cells seeded on coverslips for 30 min at 30 °C. After the incubation period, the coverslips were washed twice in PBS, fixed with 100 % methanol and observed under fluorescent microscope.
Demonstration by scanning electron microscopy
Fluorescent latex beads were incubated with harvested LRPM cells in suspension for 30 min at 30 °C in a tube (Ganassin et al. 2000). Thereafter, the cells were centrifuged at 200×g for 5 min. The pellet was washed twice with PBS. Cells from the latex bead phagocytosis assay plus control cells were subjected to 2.5 % glutaraldehyde fixation for 2 h at 4 °C. Cells were washed three times in 0.1 M phosphate buffer, each washing after 15 min of incubation at 4 °C. The cells were post-fixed with aqueous OsO4 (1 %) for 1.5 h and washed in phosphate buffer two times for 5 min to remove the unreacted fixative. The cells were dehydrated in presence of liquid nitrogen and dried. The cells were examined in Scanning Electron Microscope at University Science Instrumentation Centre, BBAU, Lucknow.
Reactive oxygen species (ROS) production
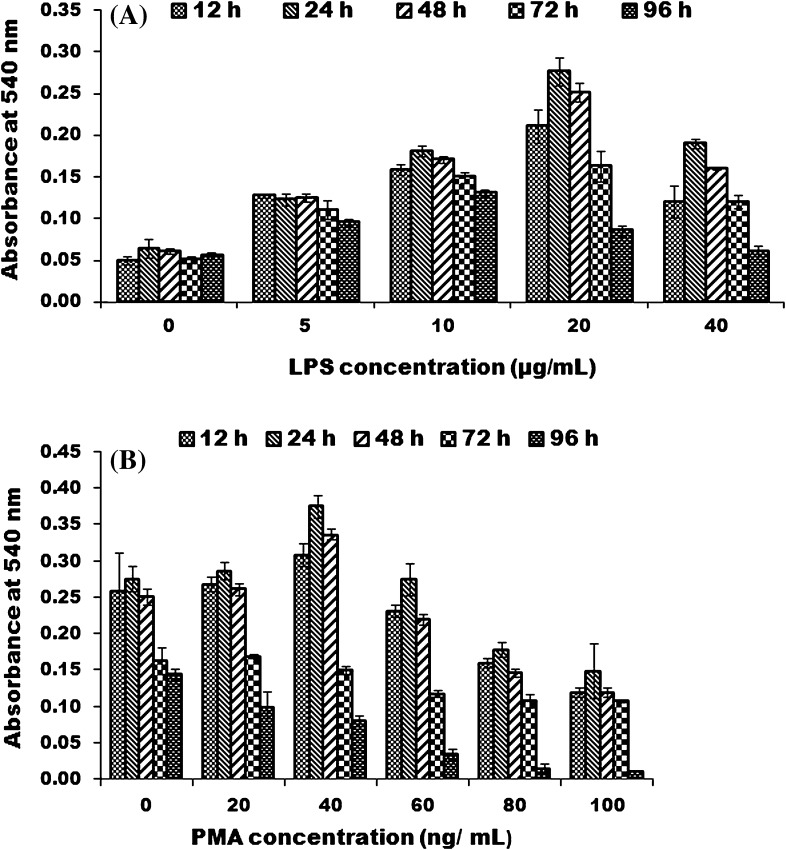
ROS production was evaluated by reduction of nitroblue tetrazolium (NBT) as described by Wang et al. (1995) with slight modifications. Hundred microliter of LRPM cells (105 cells/mL) in complete L-15 medium was seeded in each well of 96 well tissue culture plates (Nunc, Roskilde, Denmark). Cells were stimulated with different amounts of lipopolysaccharide (LPS, Sigma-Aldrich) (0–40 μg/mL) for 12, 24, 48, 72 and 96 h. After removal of the old medium, the macrophage respiratory burst was detected by covering the monolayer in each well with 100 μl of 1 mg/mL of NBT (Fermentas, Vilnius, Lithuania) in culture medium. The plates with stimulated cells were incubated for 90 min at 30 °C. The cells were then fixed with methanol, washed once with 70 % methanol to remove extracellular formazan, and the intracellular formazan was solubilized in 120 μl 2 M KOH (Sigma) and 140 μl DMSO (Merck). The plates were read at 540 nm in an ELISA reader. In another experiment, macrophages were primed with appropriate amount of LPS (20 μg/mL) for same time intervals and respiratory burst activity was triggered with different concentrations of phorbol myristate acetate (PMA, Sigma-Aldrich) (0–100 ng/mL). Rest of the procedure was same as described above.
Nitric oxide production
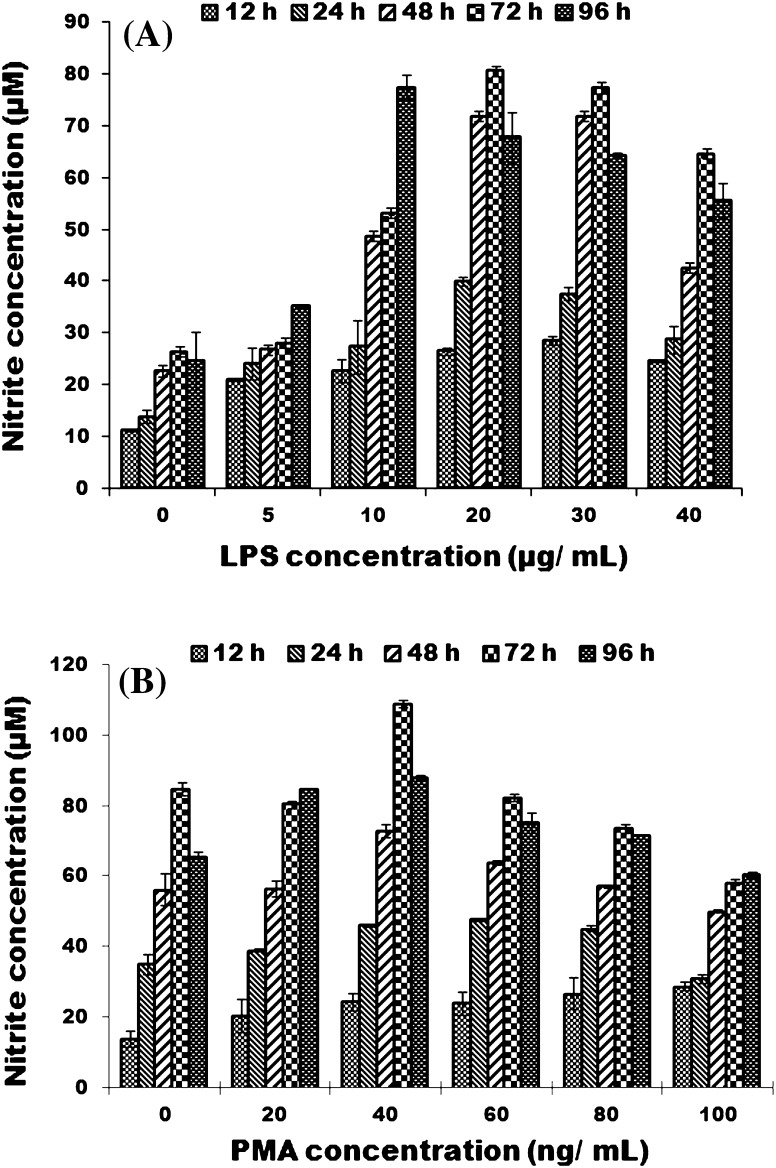
Production of nitric oxide by peritoneal macrophages was detected following the method described by Wang et al. (1995). LRPM cells were seeded into 96-well microtiter plates (1 × 105/well) and treated with complete medium having different concentrations of LPS (0–40 μg/mL) for 12, 24, 48, 72 and 96 h. In another experiment, macrophages were primed with different concentrations of PMA (0–100 ng/mL) with appropriate concentration of LPS (20 μg/mL). At different time intervals, the cell-free culture supernatants were collected in a microtitre plate and assayed for the presence of nitrite using a Nitrate/Nitrite assay kit (Amresco, Solon, USA; Cat. No. N165-kit). Nitrite assay reagents i.e. sulphanilamide and N-1-naphthylethylene diamine were mixed in a ratio 1:1 immediately before use. This assay solution was added to cell-free culture supernatants in a ratio 1:1 and mixed. The microtiter plate was incubated at room temperature for 20 min. The absorbance at 540 nm was determined in a microplate reader (Tecan, Grödig, Austria) and nitrite concentrations were determined by comparison with a standard curve prepared from known concentration of sodium nitrite.
Lysozyme assay
The lysozyme activity of LRPM was evaluated using the turbidimetric assay (Sankaran and Gurnani 1972). Briefly, a solution of 2.5 mg of Micrococcus lysodeikticus (MP Biomedicals, Mumbai, India) suspension was prepared in 10 mL of phosphate buffer (0.66 M, pH 6.4). LRPM cells were seeded into 96-well microtiter plates (1 × 105/well) and treated with complete medium having different concentrations of LPS (0–40 μg/mL) for 12, 24, 48, 72 and 96 h. In another experiment, macrophages were primed with different concentrations of PMA (0–100 ng/mL) and appropriate concentration of LPS (20 μg/mL). After different time intervals culture supernatants of LRPM cells were collected for determining lysozyme activity. For the test, 10 μl of culture supernatant was added to 290 μl of bacterial suspension in a microplate and incubated at 30 °C for 5 min. For the control, tissue culture medium L-15 was used in place of LRPM culture supernatant. The drop in turbidity (OD450) was measured after 5 min of incubation. Quantification of lysozyme activity was done as per standard definition of one unit of lysozyme. One unit of lysozyme activity corresponds to the linear decrease in OD450 of 0.001 per minute.
Cytochemistry
The smears of the LRPM cells were prepared on glass slides. These slides were stained for alpha-naphthyl acetate esterase (ANAE) activity using a kit (Sigma, Cat. No. 91-A). Briefly, the cells were fixed in citrate-acetone-methanol fixative for 30 s and washed thoroughly in deionized water. The slides were incubated in staining solution at 37 °C for 30 min. Thereafter, the slides were washed in deionized water for 2 min. The slides were air dried and examined under a microscope.
Chromosome analysis
Chromosome spreads were prepared from LRPM cells at passage 60, by conventional drop technique (Freshney 2005). The number of chromosomes in each spread was counted under a microscope.
PCR for confirmation of origin of cell line
The origin of the LRPM cell line was authenticated by partial amplification and sequencing of cytochrome c oxidase subunit 1 (COI) gene from LRPM cells following Lakra et al. (2010). Briefly, DNA was isolated from 6 × 105 LRPM cells and the COI gene was amplified and PCR product was sequenced. The obtained sequence of PCR product was compared to the known sequences of COI from L. rohita. DNA from muscle of rohu was used as positive control for PCR amplification and sequencing.
Results
Isolation and culture of peritoneal macrophages
After density gradient centrifugation of cells harvested from peritoneal cavity, an opaque ring containing MNCs was obtained at the interface. These cells after thorough washing were seeded in 25 cm2 flasks containing L-15 medium and incubated at 30 °C overnight. A well-known characteristic of macrophages and granulocytes is their capacity to adhere to glass or plastic. Macrophages started to spread over the surface within 2 h of culture, whereas neutrophilic granulocytes adhered, but remained globular after several hours in culture. The non-adherent cells were removed on the next day by washing the cells with PBS. Adherent cells showed aggregation and multiplication at several places in the flask (Fig. 1a). A complete monolayer of LRPM cells was formed in 2 weeks. Thereafter the cells were trypsinized and split in a ratio of 1:2. Subcultured cells showed faster growth than primary culture and formed a monolayer in 6 days. Gradually, a monolayer was formed within 4–5 days and after 30 passages, the monolayer was formed in 3 days at a split ratio of 1:3 (Fig. 1b).
Fig. 1.
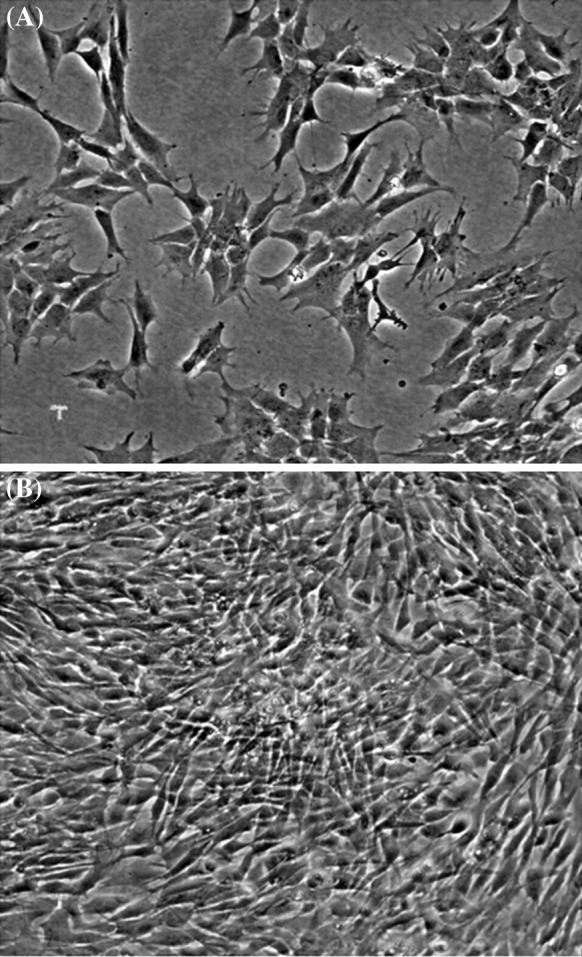
Photomicrograph of macrophages (LRPM) cells derived from peritoneal leukocytes of L. rohita. a After 2 days of plating, adherent cells were seen aggregating at some places in the flask; b Monolayer of LRPM cells at the 30th passage
Cryopreservation and revivals of cells
Cryopreserved macrophages retained their ability to proliferate in vitro and were functionally indistinguishable from non-cryopreserved macrophages. The cells revived after 6 months of storage in liquid nitrogen showed 85 % viability and grew to confluency within 2 days. There was no alteration in the morphology of cells after freezing and thawing.
Effect of temperature and FBS concentration on growth rate
Macrophage cells exhibited different growth rates at different incubation temperatures between 25 and 37 °C. However, maximum growth was obtained at 30 °C (Fig. 2a). No significant growth was observed at 25 and 37 °C. The growth rate of macrophages increased as the FBS proportion increased from 5 to 20 % at 30 °C. Cells exhibited poor growth at 5 % concentrations of FBS, relatively good growth at 10 % but maximum growth occurred at 20 % FBS concentration (Fig. 2b).
Fig. 2.
Growth curves of LRPM cells at different temperatures (a) and at different concentrations of foetal bovine serum at 30 °C (b)
Phagocytosis assay
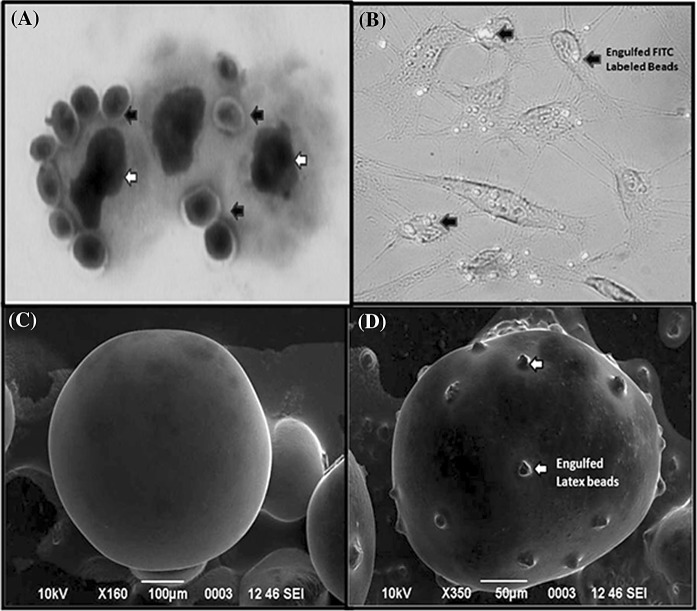
The phagocytic ability of the cultured cells was assessed by incubation with yeast cells and fluorescent latex beads. Macrophage cells actively engulfed yeasts whereas the EPC cell line did not show any phagocytic characteristics (Fig. 3a). LRPM also ingested fluorescent latex beads following incubation of cells with latex beads (Fig. 3b). A majority of LRPM cells ingested 5–6 beads though a few cells had ingested clumps of beads. The ingested fluorescent beads were observed in different planes of the phagocytizing cells. SEM analysis of cells that had been incubated with latex beads also revealed engulfment of latex beads, as seen by number of projections on the cell surface (Fig. 3d). Controls cells did not show any projections and their surface appeared smooth as compared to test cells (Fig. 3c).
Fig. 3.
Demonstration of phagocytic activity of peritoneal macrophages (LRPM) of L. rohita. Black colored arrows show yeast cells phagocytosed by LRPM cells. Numerous yeast cells were observed attached to or inside the macrophages, White colored arrows show LRPM cell nucleus (a); Phagocytosis of fluorescent latex beads by LRPM cells as seen under fluorescent microscopy in combination with bright field, Arrows indicate engulfed latex beads (b); Phagocytosis revealed by scanning electron microscopy, LRPM cells without latex beads as control shows no projections on their surface (c); Scanning electron micrograph of LRPM cells that had been incubated with latex beads. Numerous beads were observed engulfed by the macrophage (d)
Reactive oxygen species production
LRPM cells were examined for production of ROS in response to LPS and PMA at different time intervals. In one experiment, the cells were primed with varying concentration of LPS (0–40 μg/mL) for 12, 24, 48, 72 and 96 h. Maximum production of ROS was observed after 24 h with 20 μg/mL LPS (Fig. 4a). In another experiment, the cells were triggered with varying concentrations of PMA (0–100 ng/mL) and the enhanced ROS production was observed in LRPM cells after 12 h with 40 ng/mL PMA (Fig. 4b). The ROS production was much lower in wells in which no stimulant was used.
Fig. 4.
Demonstration of ROS production by LRPM cells as detected by NBT reduction assay at different time intervals. Each bar represents mean OD±SE of three wells; a Effect of different concentrations of PMA with fixed amount of LPS on ROS production; b Effect of different concentrations of PMA with a fixed amount of LPS (20 μg/mL) on ROS production
Production of nitric oxide
Nitric oxide production by peritoneal macrophage cells was examined on stimulation with different concentrations of LPS and PMA at different time intervals. The cells showed a maximum nitric oxide production in response to 20 μg/mL LPS after 72 h. After 96 h, cells with LPS 10 μg/mL production also showed approximately the same value (Fig. 5a). Combination of a concentration of PMA of 40 ng/mL and a fixed LPS (20 μg/mL) resulted in enhanced nitric oxide production in LRPM cells after 72 h (Fig. 5b).
Fig. 5.
a Demonstration of nitrite production by LRPM cells at different time intervals. Each bar represents mean nitrite concentration of three wells ±SE; a Effect of different concentrations of LPS on nitrite production; b Effect of different concentrations of PMA with fixed amount of LPS (20 μg/mL) on nitrite production
Lysozyme assay
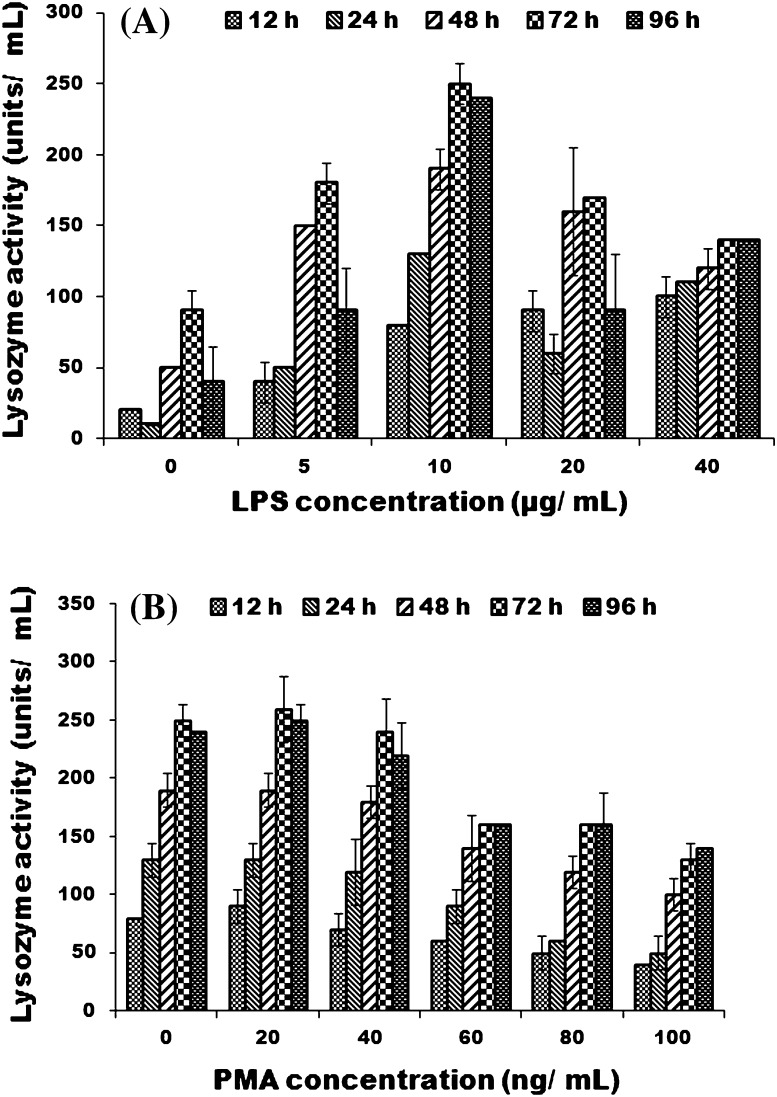
Culture supernatant from LRPM cells collected after different time intervals showed lysozyme activity. Maximum lysozyme activity was observed after 72 h in LRPM cells primed with 10 μg/mL LPS (Fig. 6a). Enhanced lysozyme activity was not observed by addition of PMA at any time duration (Fig. 6b). No lysozyme activity was detected in L-15 medium, without LPS/PMA.
Fig. 6.
Demonstration of lysozyme activity in LRPM cells at different time intervals. Each bar represents mean lysozyme activity ±SE of three wells. a Effect of different concentrations of LPS; b effect of different concentrations of PMA with a fixed amount of LPS (20 μg/mL) on lysozyme activity
Cytochemistry and chromosome analysis
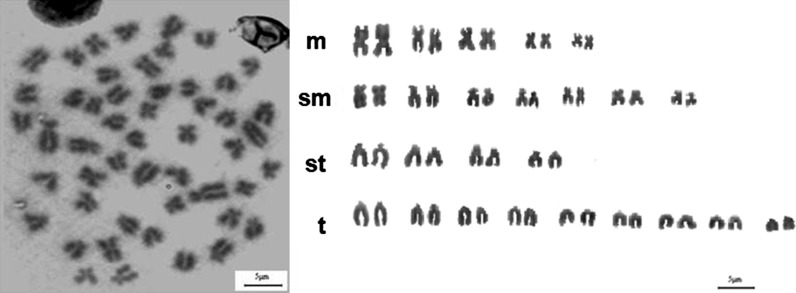
Peritoneal macrophages were moderately to strongly positive for alpha-naphthyl acetate esterase enzyme (Fig. 7). Chromosomal counts of 100 metaphase plates at passage 60 of LRPM cell line revealed that the number of chromosomes in the cells varied from 36 to 65. The majority of the cells (59 %) had a diploid chromosome number (2 N = 50) (Fig. 8).
Fig. 7.
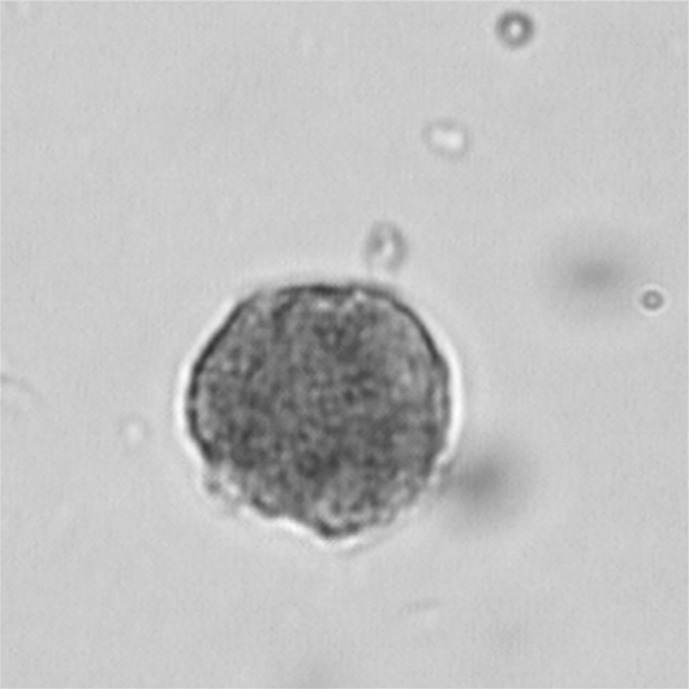
LRPM cells showing positive staining for alpha-naphthyl acetate esterase enzyme (×1,000)
Fig. 8.
Karyotype analysis of LRPM cells; a Metaphase chromosome numbers of LRPM cells at passage 60. b Karyotype of LRPM cells indicates 25 pairs of chromosomes
Confirmation of the origin of the cell line
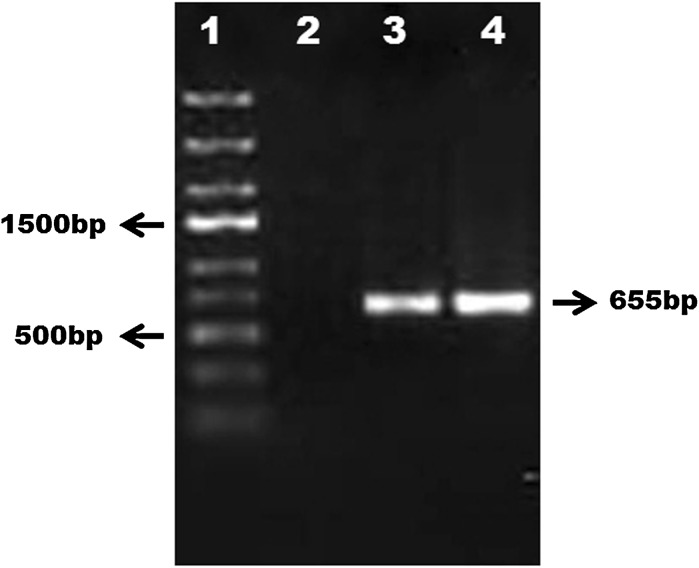
Amplification of the COI gene yielded a product of 655 bp from LRPM cells as well as rohu muscle (Fig. 9). The sequence analysis of COI fragment from LRPM showed 100 % similarity with the respective gene fragment of L. rohita (Accession No. FJ183810). The results indicated that the LRPM cells originated from L. rohita.
Fig. 9.
PCR amplification of 655 bp fragment of L. rohita genome using oligonucleotide primers from a conserved region of COI. Lane 1- Generuler express DNA ladder (Fermentas); Lane 2-COI negative control; Lane 3-Rohu muscle COI; Lane 4-LRPM COI
Discussion
One of the greatest problems in understanding immune functions of the fish is the unavailability of immune cells. In fish, macrophages are important cells in disease resistance. They are the main effector cells of the innate immune response and play a crucial role as accessory cells in both the initiation and regulation of immunity (Mulero and Meseguer 1998). In the present study, a leukocyte cell line from peritoneum of rohu has been established and characterized, which should greatly enhance the ability to study the biology of fish macrophages. Previously, long term culture of macrophages from peritoneal cavity of a marine fish, sea bream has been reported (Watanabe et al. 1997). In addition, peritoneal macrophages of channel catfish (Jenkins and Klesius 1998), sea bass (do Vale et al. 2002) and Japanese flounder (Ishibe et al. 2008) have been isolated and used for various immune studies. The LRPM cell line represents the first reported leukocyte cell line of peritoneal origin from any freshwater species of fish. The LRPM cell line has been subcultured for more than 60 passages.
The feasibility of cryopreservation of LRPM cell line was demonstrated by 85 % cell viability after thawing, and it was comparable with that reported earlier for many fish cell lines (Lakra et al. 2010; Abdul et al. 2013). The optimum growth of LRPM cells was observed at 30 °C. A number of cell lines derived from carps and other fish are known to grow best at 28–30 °C (Ishaq Ahmed et al. 2009; Lakra et al. 2010). The maximum growth of cells was observed in L-15 medium supplemented with 20 % FBS. However, even 10 and 15 % FBS concentration in L-15 medium also resulted in relatively good growth, and hence, 10 % can be used for growth and maintenance of this cell line at low cost.
Phagocytosis, a fundamental defense mechanism in most animal species including fish, is mediated by phagocytic cells such as neutrophils, monocytes and macrophages (Ishibe et al. 2008). Phagocytic cells of the monocytic–macrophage lineage play a dominant role in the defense against invading pathogenic bacteria in fish (Neumann et al. 2000; Joerink et al. 2006; Cerezuela et al. 2009). Macrophage phagocytosis has also been used as an immunological parameter to evaluate the health/immune function of different fish species under different biotic and abiotic factors (Jensch-Junior et al. 2006). Peritoneal macrophages are known to exhibit immune and non-immune phagocytosis under in vitro conditions (Jensch-Junior et al. 2006; Ishibe et al. 2008). LRPM cells showed avid phagocytosis of yeast cells and latex beads as shown by light and electron microscopy. SEM has been previously used to validate phagocytosis by kidney phagocytes (Norum et al. 2005). Demonstration of phagocytosis by LRPM cells is an important parameter of differentiating a macrophage cell line from other cell lines.
Antibacterial defense mechanism of macrophages is mediated by the production of ROS, NO and lysozyme. No studies are available concerning the effects of time duration on immune functions like ROS, nitric oxide production and lysozyme activity. Fish macrophages engulf invading pathogens and destroy them by producing ROS such as superoxide and their metabolites. The quantity of ROS released serves as an indicator of the innate immune responses and general health of fish (Hermann and Kim 2005; Datta et al. 2009). This oxygen-dependent bactericidal mechanism has been demonstrated in phagocytes of many different fish species (Ishibe et al. 2008; Paredes et al. 2013). Optimum concentration of LPS is a strong stimulator of macrophages as it activates several signal transduction pathways to produce a variety of inflammatory cytokines in humans and animals including fish. In this study, LRPM produced high ROS in response to LPS stimulation after 24 h, but combination of LPS and PMA showed early and enhanced production after 12 h. On the other hand, Sarmento et al. (2004) found an initial increase in ROS by LPS-stimulated macrophages followed by down-regulation after 24 h of incubation.
Nitric oxide has been shown to have potent antimicrobial effects against a number of relevant fish pathogens (Hanington and Belosevic 2007; Forlenza et al. 2009). Nitric oxide production is stated to be enhanced by LPS-stimulated fish macrophages (Neumann et al. 1995). LRPM cells produced maximum amount of nitric oxide upon stimulation with 20 μg/mL of LPS after 72 h. Higher production of nitric oxide was observed in combination of LPS with PMA. The nitric oxide activity of fish macrophages has been demonstrated previously in nurse shark (Walash et al. 2006), rainbow trout (Campos-Perez et al. 2000) and turbot (Tafalla and Novoa 2000). The ability of fish macrophages to synthesize nitric oxide suggests that they share the same effector molecules for microbicidal and tumoricidal activities with mammalian macrophages.
Lysozyme is an important defense molecule of the innate immune system, playing a role in mediating protection against microbial invasion. It is a mucolytic enzyme produced by leucocytes, especially macrophages. Fish lysozyme possesses lytic activity against bacteria and can activate complement and phagocytes (Alvarez-Pellitero 2008). Priming of LRPM cells with LPS shows maximum increase in lysozyme activity after 72 h. Addition of PMA did not show any significant effect on lysozyme activity of LRPM. Lysozyme activity has been previously shown by fish macrophages (Chaudhary et al. 2012b; Kadowaki et al. 2013). But, no studies are available regarding the synergetic effect of LPS and PMA on lysozyme activity in other fish macrophages.
Monocytes and macrophages stain brown under α-naphthyl esterase staining. Studies have shown that the diffuse reaction product of non-specific esterase in the cytoplasm of monocytes/macrophages can be readily distinguished from the discrete granular non-specific esterase staining in granulocytes and lymphocytes of fish leucocytes (Tumbol et al. 2009). The cultured cells were positive for alpha-naphthyl acetate esterase. Esterase staining is regarded as the most reliable cytochemical marker for mammalian macrophages (Gallily and Savion 1983). Similarly, macrophages from fish species have also been found to be positive for esterase (Jorgensen et al. 1993; Wang et al. 1995). The origin of the LRPM cell line was authenticated by partial amplification and sequencing of COI gene of the rohu. The mitochondrial COI gene sequence alignment has been used as reliable molecular method to accurately identify the origin of cell lines of many fish species, such as rohu (Lakra et al. 2010) and red-line torpedo (Swaminathan et al. 2012).
In conclusion, the LRPM cells have morphological and functional characteristics of macrophages. The developed cell line will be useful in studying pathogen–macrophage interaction and in studying the role of macrophages in non-specific immune response. Moreover, the LRPM cell line can be a potential source of fish-specific enzymes and cytokines. The leukocyte cell line characterized in the present study will be an invaluable tool for evaluating the efficacy of aquaculture drugs, and also for studying the impact of chemicals/pollutants used in aquaculture.
Acknowledgments
The authors are thankful to Dr J.K. Jena, Director, NBFGR, Lucknow and Dr P. Punia, HOD, FHM Division, NBFGR, Lucknow for their guidance and encouragement. We are also thankful to Dr V. Elangovan, Coordinator, University Science Instrumentation Centre, BBAU, Lucknow for his help in electron microscopy.
References
- Abdul MS, Nambi KS, Taju G, Sundar RN, Madan N, Sahul Hameed AS. Establishment and characterization of permanent cell line from gill tissue of Labeo rohita (Hamilton) and its application in gene expression and toxicology. Cell Biol Toxicol. 2013;29:59–73. doi: 10.1007/s10565-012-9237-7. [DOI] [PubMed] [Google Scholar]
- Alvarez-Pellitero P. Fish immunity and parasite infections: from innate immunity to immunoprophylactic prospects. Vet Immunol Immunopathol. 2008;126:173–198. doi: 10.1016/j.vetimm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Campos-Perez JJ, Ward M, Grabowski PS, Ellis AE, Secombes CJ. The gills are an important site of iNOS expression in rainbow trout Oncorhynchus mykiss after challenge with the gram-positive pathogen Renibacterium salmoninarum. Immunology. 2000;99:153–161. doi: 10.1046/j.1365-2567.2000.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezuela R, Cuesta A, Meseguer J, Angeles Esteban M. Effects of dietary vitamin D3 administration on innate immune parameters of seabream (Sparus aurata L.) Fish Shellfish Immunol. 2009;26:243–248. doi: 10.1016/j.fsi.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Chaudhary DK, Sood N, Pradhan PK, Singh A, Punia P, Agarwal NK, Rathore G. Establishment of a macrophage cell line from adherent peripheral blood mononuclear cells of Catla catla. In Vitro Cell Dev Biol Anim. 2012;48:340–348. doi: 10.1007/s11626-012-9516-x. [DOI] [PubMed] [Google Scholar]
- Chaudhary DK, Sood N, Rathore G, Pradhan PK, Punia P, Agarwal NK, Jena JK. Establishment and characterization of a macrophage cell line from thymus of Catla catla (Hamilton, 1822) Aquac Res. 2012 doi: 10.1016/j.gene.2012.09.081. [DOI] [PubMed] [Google Scholar]
- Datta S, Ghosh D, Saha DR, Bhattacharya S, Mazumder S. Chronic exposure to low concentration of arsenic is immunotoxic to fish: role of head kidney macrophages as biomarkers of arsenic toxicity of Clarias batrachus. Aquat Toxicol. 2009;92:86–94. doi: 10.1016/j.aquatox.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Dewitte-Orr SJ, Lepic K, Bryson SP, Walsh SK, Lee LE, Bols NC. Development of a continuous cell line, PBLE, from an American eel peripheral blood leukocyte preparation. In Vitro Cell Dev Biol Anim. 2006;42:263–272. doi: 10.1290/0604023.1. [DOI] [PubMed] [Google Scholar]
- do Vale A, Afonso A, Silva MT. The professional phagocytes of sea bass (Dicentrarchus labrax L.): cytochemical characterisation of neutrophils and macrophages in the normal and inflamed peritoneal cavity. Fish Shellfish Immunol. 2002;13:183–198. doi: 10.1006/fsim.2001.0394. [DOI] [PubMed] [Google Scholar]
- Forlenza M, Nakao M, Wibowo I, Joerink M, Arts JA, Savelkoul HF, Wiegertjes GF. Nitric oxide hinders antibody clearance from the surface of Trypanoplasma borreli and increases susceptibility to complement-mediated lysis. Mol Immunol. 2009;46:3188–3197. doi: 10.1016/j.molimm.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Freshney RI. Culture of animal cells: a manual of basic technique. New Jersy: Wiley; 2005. [Google Scholar]
- Gallily R, Savion N. Cultivation, proliferation and characterization of thymic macrophages. Immunology. 1983;50:139–148. [PMC free article] [PubMed] [Google Scholar]
- Ganassin RC, Schirmer K, Bols NC. Cell and Tissue Research. In: Ostrander GK, editor. The laboratory fish. San Diego: Academic Press; 2000. pp. 631–651. [Google Scholar]
- Ghosn EE, Cassado AA, Govoni GR, Fukuhara T, Yang Y, Monack DM, Bortoluci KR, Almeida SR, Herzenberg LA. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci USA. 2010;107:2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanington PC, Belosevic M. Interleukin-6 family cytokine M17 induces differentiation and nitric oxide response of goldfish (Carassius auratus L.) macrophages. Dev Comp Immunol. 2007;31:817–829. doi: 10.1016/j.dci.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Hermann AC, Kim CH. Effects of arsenic on zebrafish innate immune system. Mar Biotechnol. 2005;7:494–505. doi: 10.1007/s10126-004-4109-7. [DOI] [PubMed] [Google Scholar]
- Ishaq Ahmed VP, Babu VS, Chandra V, Nambi KS, Thomas J, Bhonde R, Sahul Hameed AS. A new fibroblastic-like cell line from heart muscle of the Indian major carp (Catla catla): development and characterization. Aquaculture. 2009;293:180–186. doi: 10.1016/j.aquaculture.2009.05.012. [DOI] [Google Scholar]
- Ishibe K, Osatomi K, Hara K, Kanai K, Yamaguchi K, Oda T. Comparison of the responses of peritoneal macrophages from Japanese flounder (Paralichthys olivaceus) against high virulent and low virulent strains of Edwardsiella tarda. Fish Shellfish Immunol. 2008;24:243–251. doi: 10.1016/j.fsi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Jenkins JA, Klesius PH. Elicitation of Macrophages from the peritoneal cavity of Channel catfish. J Aquat Anim Health. 1998;10:69–74. doi: 10.1577/1548-8667(1998)010<0069:EOMFTP>2.0.CO;2. [DOI] [Google Scholar]
- Jensch-Junior BE, Pressinotti LN, Borges JCS, deSilva JRMC. Characterization of macrophage phagocytosis of the tropical fish Prochilodus scrofa (Steindachner, 1881) Aquaculture. 2006;251:509–515. doi: 10.1016/j.aquaculture.2005.05.042. [DOI] [Google Scholar]
- Joerink M, Ribeiro CM, Stet RJ, Hermsen T, Savelkoul HF, Wiegertjes GF. Head kidney-derived macrophages of common carp (Cyprinus carpio L.) show plasticity and functional polarization upon differential stimulation. J Immunol. 2006;177:61–69. doi: 10.4049/jimmunol.177.1.61. [DOI] [PubMed] [Google Scholar]
- Jorgensen JB, Lunde H, Robertsen B. Peritoneal and head kidney cell response to intraperitoneally injected yeast glucan in Atlantic salmon, Salmo salar L. J Fish Dis. 1993;16:313–325. doi: 10.1111/j.1365-2761.1993.tb00865.x. [DOI] [Google Scholar]
- Kadowaki T, Yasui Y, Nishimiya O, Takahashi Y, Kohchi C, Soma G, Inagawa H. Orally administered LPS enhances head kidney macrophage activation with down-regulation of IL-6 in common carp (Cyprinus carpio) Fish Shellfish Immunol. 2013;34:1569–1575. doi: 10.1016/j.fsi.2013.03.372. [DOI] [PubMed] [Google Scholar]
- Lakra WS, Swaminathan TR, Rathore G, Goswami M, Yadav K, Kapoor S. Development and characterization of three new diploid cell lines from Labeo rohita (Ham.) Biotechnol Prog. 2010;26:1008–1013. doi: 10.1002/btpr.418. [DOI] [PubMed] [Google Scholar]
- McCarthy UM, Bron JE, Brown L, Pourahmad F, Bricknell IR, Thompson KD, Adams A, Ellis AE. Survival and replication of Piscirickettsia salmonis in rainbow trout head kidney macrophages. Fish Shellfish Immunol. 2008;25:477–484. doi: 10.1016/j.fsi.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Mulero V, Meseguer J. Functional characterisation of a macrophage-activating factor produced by leucocytes of gilt-head seabream (Sparus aurata L.) Fish Shellfish Immunol. 1998;8:143–156. doi: 10.1006/fsim.1997.0127. [DOI] [Google Scholar]
- Neumann NF, Fagan D, Belosevic M. Macrophage activating factor(s) secreted by mitogen stimulated goldfish kidney leukocytes synergize with bacterial lipopolysaccharide to induce nitric oxide production in teleost macrophages. Dev Comp Immunol. 1995;19:473–482. doi: 10.1016/0145-305X(95)00032-O. [DOI] [PubMed] [Google Scholar]
- Neumann NF, Barreda DR, Belosevic M. Generation and functional analysis of distinct macrophage sub-populations from goldfish (Carassius auratus L.) kidney leukocyte cultures. Fish Shellfish Immunol. 2000;10:1–20. doi: 10.1006/fsim.1999.0221. [DOI] [PubMed] [Google Scholar]
- Norum M, Bogwald J, Dalmo RA. Isolation and characterisation of spotted wolffish (Anarhichas minor Olafsen) macrophages. Fish Shellfish Immunol. 2005;18:381–391. doi: 10.1016/j.fsi.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Paredes M, Gonzalez K, Figueroa J, Montiel-Eulefi E (2013) Immunomodulatory effect of prolactin on Atlantic salmon (Salmo salar) macrophage function. Fish Physiol Biochem 39:1215–1221 [DOI] [PubMed]
- Roy B, Rai U. Dual mode of catecholamine action on splenic macrophage phagocytosis in wall lizard, Hemidactylus flaviviridis. Gen Comp Endocrinol. 2004;136:180–191. doi: 10.1016/j.ygcen.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Russo R, Shoemaker CA, Panangala VS, Klesius PH. In vitro and in vivo interaction of macrophages from vaccinated and non-vaccinated channel catfish (Ictalurus punctatus) to Edwardsiella ictaluri. Fish Shellfish Immunol. 2009;26:543–552. doi: 10.1016/j.fsi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Sankaran K, Gurnani S. On the variation in the catalytic activity of lysozyme in fishes. Indian J Biochem Biophys. 1972;9:162–165. [PubMed] [Google Scholar]
- Sarmento A, Marques F, Anthony E, Ellis AE, Afonso A. Modulation of the activity of sea bass (Dicentrarchus labrax) head kidney macrophages by macrophage activating factor(s) and lipopolysaccharide. Fish Shellfish Immunol. 2004;16:79–92. doi: 10.1016/S1050-4648(03)00031-7. [DOI] [PubMed] [Google Scholar]
- Secombes CJ, Fletcher TC. The role of phagocytes in the protective mechanisms of fish. Annu Rev Fish Dis. 1992;2:53–71. doi: 10.1016/0959-8030(92)90056-4. [DOI] [Google Scholar]
- Sunyer JO. Fishing for mammalian paradigms in the teleost immune system. Nat Immunol. 2013;14:320–326. doi: 10.1038/ni.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan TR, Lakra WS, Gopalakrishnan A, Basheer VS, Kushwaha B, Sajeela K. Development and characterization of a fibroblastic-like cell line from caudal fin of the red-line torpedo, Puntius denisonii (Day) (Teleostei: Cyprinidae) Aquacult Res. 2012;43:498–508. doi: 10.1111/j.1365-2109.2011.02854.x. [DOI] [Google Scholar]
- Tafalla C, Novoa B. Requirements for nitric oxide production by turbot (Scophthalmus maximus) head kidney macrophages. Dev Comp Immunol. 2000;24:623–631. doi: 10.1016/S0145-305X(99)00087-7. [DOI] [PubMed] [Google Scholar]
- Tumbol RA, Baiano JC, Barnes AC. Differing cell population structure reflects differing activity of Percoll-separated pronephros and peritoneal leucocytes from barramundi (Lates calcarifer) Aquaculture. 2009;292:180–188. doi: 10.1016/j.aquaculture.2009.04.030. [DOI] [Google Scholar]
- Vallejo AN, Ellsaesser CF, Miller NW, Clem LW. Spontaneous development of functionally active long-term monocyte like cell lines from channel catfish. In Vitro Cell Dev Biol Anim. 1991;27:279–286. doi: 10.1007/BF02630904. [DOI] [PubMed] [Google Scholar]
- Walash CJ, Toranto JD, Gilliland CT, Noyes DR, Bodine AB, Luer CA. Nitric oxide production by nurse shark (Ginglymostoma cirratum) and clearnose skate (Raja eglanteria) peripheral blood leucocytes. Fish Shellfish Immunol. 2006;20:40–46. doi: 10.1016/j.fsi.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Wang R, Neumann NF, Shen Q, Belosevic M. Establishment and characterization of a macrophage cell line from the goldfish. Fish Shellfish Immunol. 1995;5:329–346. doi: 10.1006/fsim.1995.0032. [DOI] [Google Scholar]
- Watanabe T, Shoho T, Ohta H, Kubo N, Kono M, Furukawa K. Long-term cell culture of resident peritoneal macrophages from red sea bream Pagrus major. Fish Sci. 1997;63:862–866. [Google Scholar]
- Weyts FAA, Rombout JHWM, Verburg-Kemenade BML. A common carp (Cyprinus carpio L.) leukocyte cell line shares morphological and functional characteristics with macrophages. Fish Shellfish Immunol. 1997;7:123–133. doi: 10.1006/fsim.1996.0069. [DOI] [Google Scholar]
- Zhang X, Goncalves R, Mosser D M (2008) The isolation and characterization of murine macrophages. Curr Prot Immunol Chapter 14: Unit 14.1. doi:10.1002/0471142735.in1401s83 [DOI] [PMC free article] [PubMed]