Table 2.
Multidisciplinary Evaluation Characteristics of Patients With HCC Seen at MEDVAMC by Consult Type (N = 116)
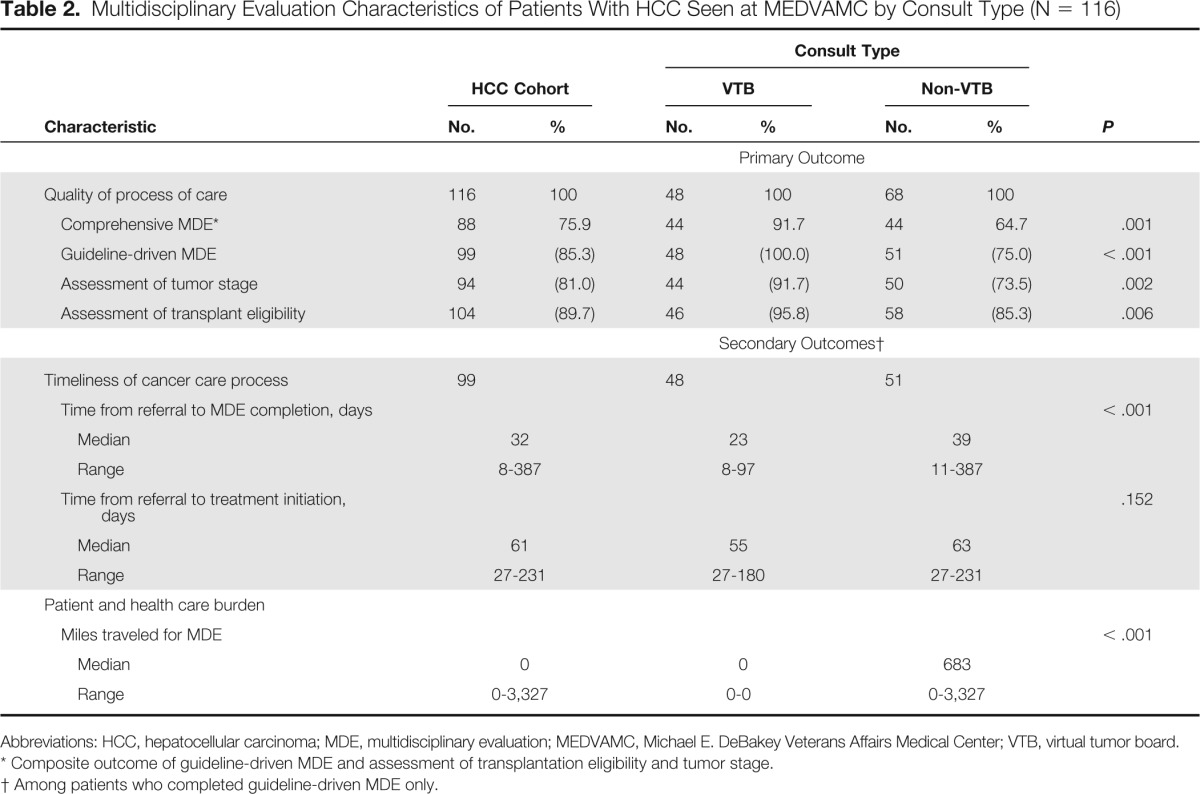
| Characteristic | HCC Cohort |
Consult Type |
P | ||||
|---|---|---|---|---|---|---|---|
| VTB |
Non-VTB |
||||||
| No. | % | No. | % | No. | % | ||
| Primary Outcome | |||||||
| Quality of process of care | 116 | 100 | 48 | 100 | 68 | 100 | |
| Comprehensive MDE* | 88 | 75.9 | 44 | 91.7 | 44 | 64.7 | .001 |
| Guideline-driven MDE | 99 | (85.3) | 48 | (100.0) | 51 | (75.0) | < .001 |
| Assessment of tumor stage | 94 | (81.0) | 44 | (91.7) | 50 | (73.5) | .002 |
| Assessment of transplant eligibility | 104 | (89.7) | 46 | (95.8) | 58 | (85.3) | .006 |
| Secondary Outcomes† | |||||||
| Timeliness of cancer care process | 99 | 48 | 51 | ||||
| Time from referral to MDE completion, days | < .001 | ||||||
| Median | 32 | 23 | 39 | ||||
| Range | 8-387 | 8-97 | 11-387 | ||||
| Time from referral to treatment initiation, days | .152 | ||||||
| Median | 61 | 55 | 63 | ||||
| Range | 27-231 | 27-180 | 27-231 | ||||
| Patient and health care burden | |||||||
| Miles traveled for MDE | < .001 | ||||||
| Median | 0 | 0 | 683 | ||||
| Range | 0-3,327 | 0-0 | 0-3,327 | ||||
Abbreviations: HCC, hepatocellular carcinoma; MDE, multidisciplinary evaluation; MEDVAMC, Michael E. DeBakey Veterans Affairs Medical Center; VTB, virtual tumor board.
Composite outcome of guideline-driven MDE and assessment of transplantation eligibility and tumor stage.
Among patients who completed guideline-driven MDE only.
