Abstract
After being paired with females, male prairie voles show major changes in their social behaviors among which is an increase in paternal responsiveness. These changes are accompanied by fluctuations in the density of the [Arg8]vasopressin-immunoreactive (AVP-ir) fibers in the lateral septum, suggesting that septal AVP might be involved in these changes. To explore a possible involvement of septal AVP in paternal responsiveness, we tested whether injections of saline, AVP, or the V1a receptor antagonist [1-(beta-mercapto-beta, beta-cyclopentamethylenepropionic acid),2-(O-methyltyrosine]AVP [d(CH2)5Tyr(Me)AVP] into the lateral septum influenced the four most prominent paternal activities displayed by male prairie voles; grooming, crouching over, contacting, and retrieving pups. In a first experiment, sexually inexperienced males received a single injection of AVP, saline, or d(CH2)5Tyr(Me)AVP in the lateral septum, after which their paternal responsiveness was recorded during a 10-min period. AVP-injected animals spent more time contacting and crouching over pups, while d(CH2)5Tyr(Me)AVP-injected animals spent less time grooming pups than saline-injected animals. In a follow-up study, one group of animals received an injection of AVP preceded by an injection of saline or d(CH2)5Tyr(Me)-AVP into the lateral septum. A second group of animals received an injection of saline preceded by an injection of saline or d(CH2)5Tyr(Me)AVP into the lateral septum. In both groups, animals spent less time grooming, crouching over, and contacting pups if they had first been injected with d(CH2)5Tyr(Me)AVP. Control experiments suggested that the effects of AVP on paternal responsiveness were dose- and site-specific. These data suggest that septal AVP enhances paternal responsiveness by a V1a receptor-mediated mechanism.
Full text
PDF
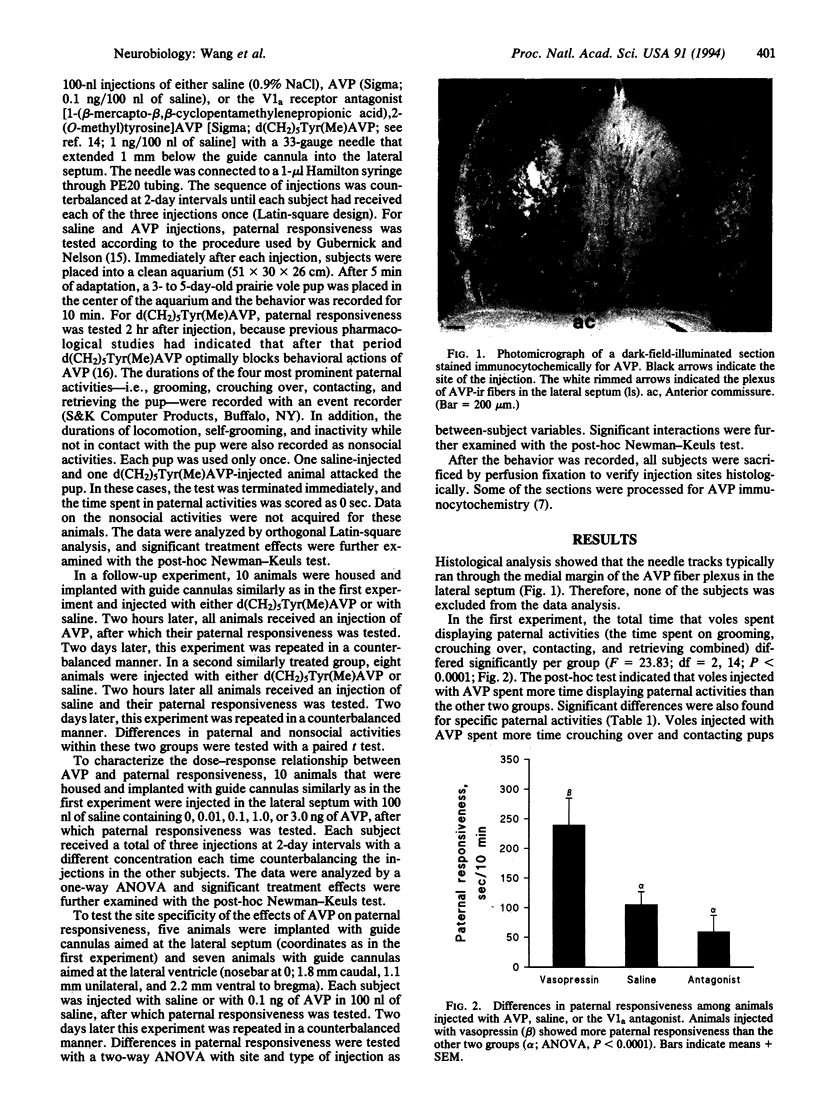
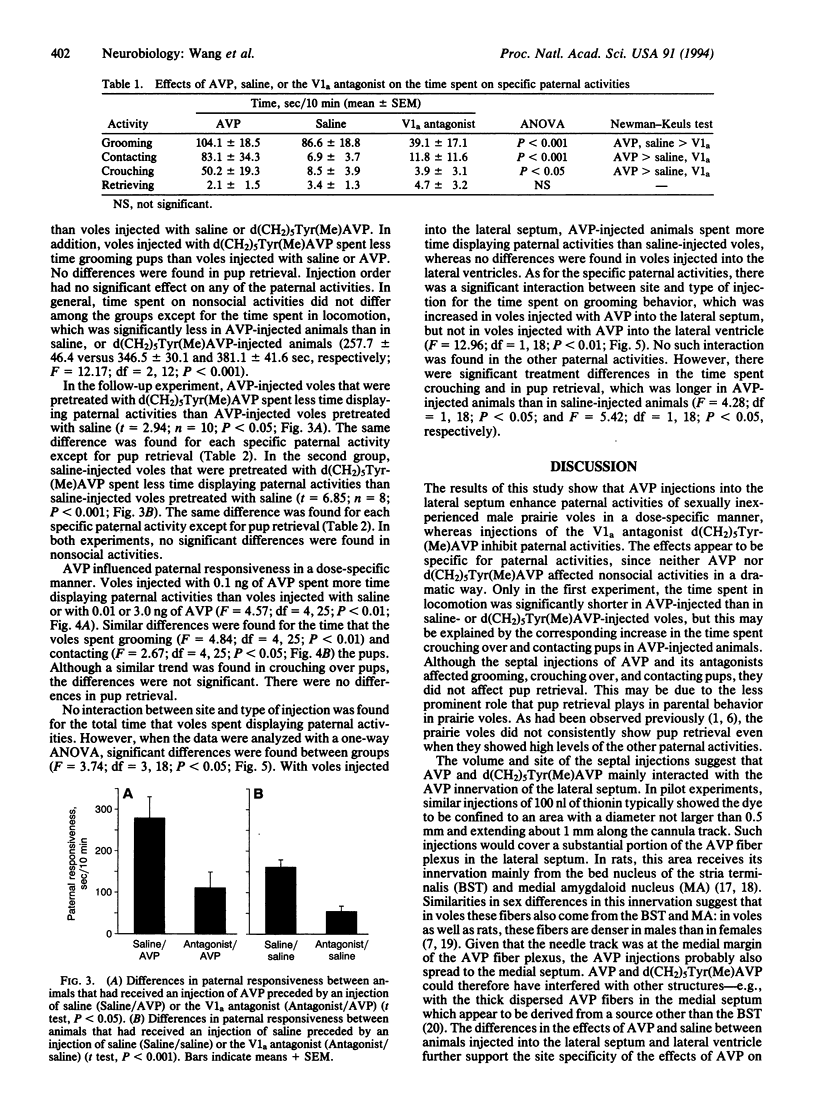
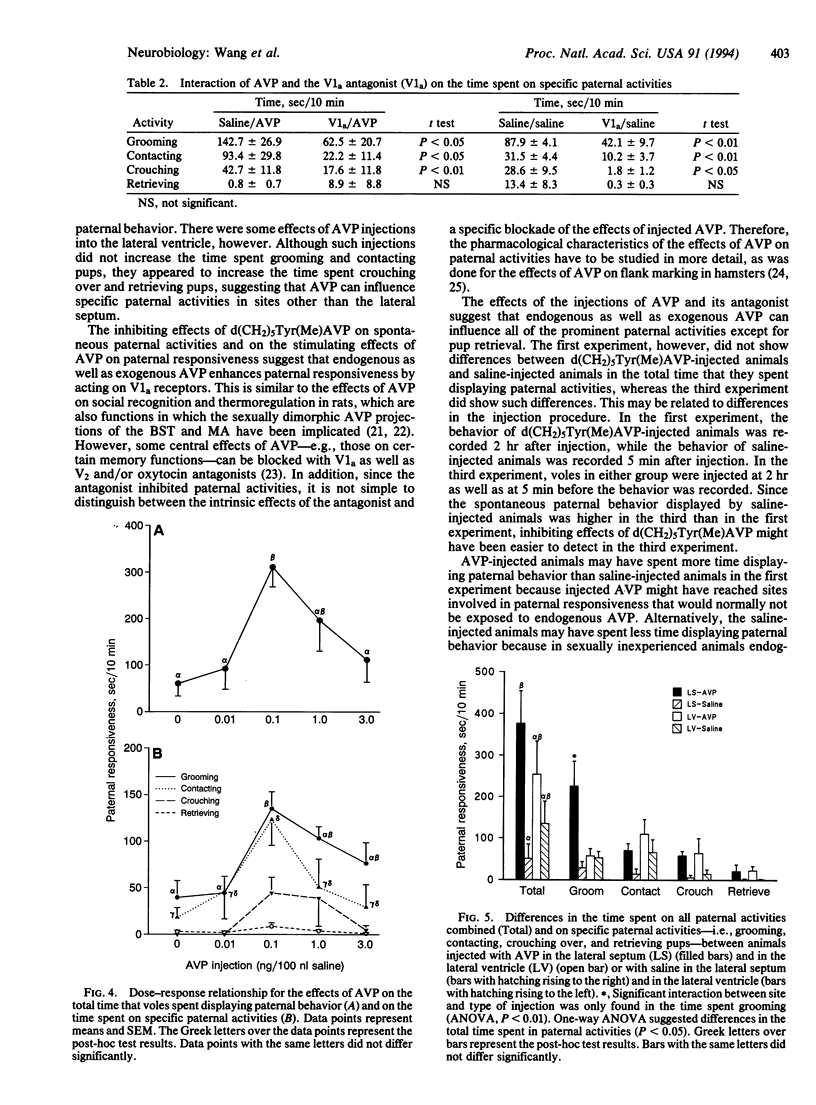

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers H. E., Pollock J., Simmons W. H., Ferris C. F. A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J Neurosci. 1986 Jul;6(7):2085–2089. doi: 10.1523/JNEUROSCI.06-07-02085.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M., Novak M. A., De Vries G. J. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. J Neuroendocrinol. 1993 Jun;5(3):247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Baverstock P., Green B. Water recycling in lactation. Science. 1975 Feb 21;187(4177):657–658. doi: 10.1126/science.1167701. [DOI] [PubMed] [Google Scholar]
- Bluthe R. M., Schoenen J., Dantzer R. Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res. 1990 Jun 11;519(1-2):150–157. doi: 10.1016/0006-8993(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Caffé A. R., van Leeuwen F. W., Luiten P. G. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987 Jul 8;261(2):237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- Crenshaw B. J., De Vries G. J., Yahr P. Vasopressin innervation of sexually dimorphic structures of the gerbil forebrain under various hormonal conditions. J Comp Neurol. 1992 Aug 22;322(4):589–598. doi: 10.1002/cne.903220412. [DOI] [PubMed] [Google Scholar]
- De Vries G. J., Buijs R. M. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983 Aug 29;273(2):307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- DeVries G. J., Buijs R. M., Van Leeuwen F. W., Caffé A. R., Swaab D. F. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985 Mar 8;233(2):236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- Demotes-Mainard J., Chauveau J., Rodriguez F., Vincent J. D., Poulain D. A. Septal release of vasopressin in response to osmotic, hypovolemic and electrical stimulation in rats. Brain Res. 1986 Sep 3;381(2):314–321. doi: 10.1016/0006-8993(86)90082-x. [DOI] [PubMed] [Google Scholar]
- Ferris C. F., Pollock J., Albers H. E., Leeman S. E. Inhibition of flank-marking behavior in golden hamsters by microinjection of a vasopressin antagonist into the hypothalamus. Neurosci Lett. 1985 Apr 9;55(2):239–243. doi: 10.1016/0304-3940(85)90027-8. [DOI] [PubMed] [Google Scholar]
- Ferris C. F., Singer E. A., Meenan D. M., Albers H. E. Inhibition of vasopressin-stimulated flank marking behavior by V1-receptor antagonists. Eur J Pharmacol. 1988 Sep 13;154(2):153–159. doi: 10.1016/0014-2999(88)90092-1. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Slotnick B. M. Disruption of maternal behavior in rats with lesions of the septal area. Physiol Behav. 1978 Aug;21(2):189–200. doi: 10.1016/0031-9384(78)90041-0. [DOI] [PubMed] [Google Scholar]
- Friedman M. I., Bruno J. P. Letter: Exchange of water during lactation. Science. 1976 Jan 30;191(4225):409–410. doi: 10.1126/science.1246627. [DOI] [PubMed] [Google Scholar]
- Gubernick D. J., Nelson R. J. Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm Behav. 1989 Jun;23(2):203–210. doi: 10.1016/0018-506x(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Shapiro L. E. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans J. E., Leon M. The effects of lactation and ambient temperature on the body temperature of female Norway rats. Physiol Behav. 1983 Jun;30(6):959–961. doi: 10.1016/0031-9384(83)90261-5. [DOI] [PubMed] [Google Scholar]
- Kruszynski M., Lammek B., Manning M., Seto J., Haldar J., Sawyer W. H. [1-beta-Mercapto-beta,beta-cyclopentamethylenepropionic acid),2-(O-methyl)tyrosine ]argine-vasopressin and [1-beta-mercapto-beta,beta-cyclopentamethylenepropionic acid)]argine-vasopressine, two highly potent antagonists of the vasopressor response to arginine-vasopressin. J Med Chem. 1980 Apr;23(4):364–368. doi: 10.1021/jm00178a003. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Malkinson T. J., Veale W. L., Lederis K., Pittman Q. J. Vasopressin and oxytocin in rat brain in response to prostaglandin fever. Am J Physiol. 1990 Nov;259(5 Pt 2):R1056–R1062. doi: 10.1152/ajpregu.1990.259.5.R1056. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Neumann I., Schwarzberg H. Central and peripheral release of vasopressin and oxytocin in the conscious rat after osmotic stimulation. Brain Res. 1988 Aug 9;457(2):219–225. doi: 10.1016/0006-8993(88)90689-0. [DOI] [PubMed] [Google Scholar]
- Mayes C. R., Watts A. G., McQueen J. K., Fink G., Charlton H. M. Gonadal steroids influence neurophysin II distribution in the forebrain of normal and mutant mice. Neuroscience. 1988 Jun;25(3):1013–1022. doi: 10.1016/0306-4522(88)90054-1. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Urban J. H., Dorsa D. M. Steroid dependency of vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Endocrinology. 1989 Nov;125(5):2335–2340. doi: 10.1210/endo-125-5-2335. [DOI] [PubMed] [Google Scholar]
- Numan M. Neural basis of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13(1-2):47–62. doi: 10.1016/0306-4530(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Pedersen C. A., Ascher J. A., Monroe Y. L., Prange A. J., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982 May 7;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pittman Q. J., Malkinson T. J., Kasting N. W., Veale W. L. Enhanced fever following castration: possible involvement of brain arginine vasopressin. Am J Physiol. 1988 Mar;254(3 Pt 2):R513–R517. doi: 10.1152/ajpregu.1988.254.3.R513. [DOI] [PubMed] [Google Scholar]
- Platchkov S, Amroun A, Bricault P, Cavedon JM, de Witt Huberts PK, Dreux P, Frois B, Goodman CD, Goutte D, Martino J. Measurement of the 1f7/2-neutron-orbit radius in 41Ca. Phys Rev Lett. 1988 Sep 26;61(13):1465–1468. doi: 10.1103/PhysRevLett.61.1465. [DOI] [PubMed] [Google Scholar]
- Slotnick B. M., Nigrosh B. J. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J Comp Physiol Psychol. 1975 Jan;88(1):118–127. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- Winslow J. T., Hastings N., Carter C. S., Harbaugh C. R., Insel T. R. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993 Oct 7;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- de Vries G. J., Buijs R. M., Swaab D. F. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain--presence of a sex difference in the lateral septum. Brain Res. 1981 Aug 10;218(1-2):67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- de Wied D., Elands J., Kovács G. Interactive effects of neurohypophyseal neuropeptides with receptor antagonists on passive avoidance behavior: mediation by a cerebral neurohypophyseal hormone receptor? Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1494–1498. doi: 10.1073/pnas.88.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]




