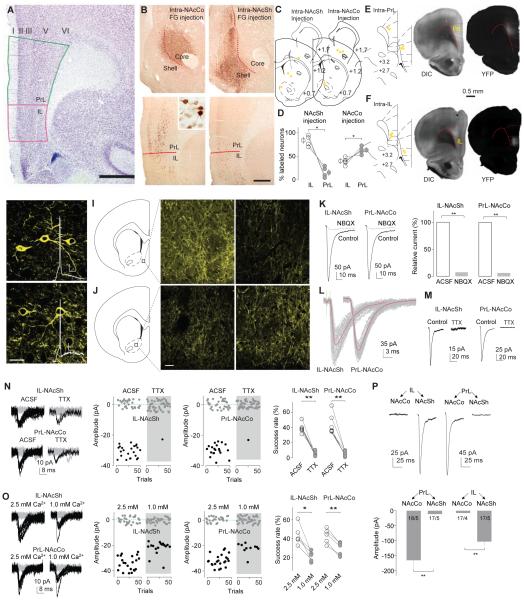
Figure 1. Anatomical and electrophysiological differentiation of the IL-to-NAcSh and PrL-to-NAcCo projections in rats.
A A coronal section stained for Nissl showing the cytoarchitectonic boundaries of the IL and PrL in the rat, including the relatively indistinct lamina in the IL (but not the PrL) and the sudden change from a compacted to a scattered layer II at the PrL-IL border. Scale bar, 1 mm. B Representative images showing immunoperoxidase labeling for FG at the injection sites involving mainly the NAcCo or NAcSh and the corresponding distribution of retrogradely labeled cells in the PrL and IL. Insert shows FG-labeled neurons at 40x magnification. Scale bar, 500 μm; 50 μm for insert. C Diagrams of coronal slices showing the injection sites (yellow dots) of FG in the NAcCo of 5 rats (left) and ventral-medial NAcSh of 4 rats (right). D Summarized results showing that intra-NAcSh injections of FG resulted in a higher percentage of labeled neurons in IL (83.1±5.9%) than PrL (16.9±5.9%; t3=5.65, p=0.01), and that intra-NAcCo injection of FG resulted in a higher percentage of neurons labeled in PrL (61.7±3.2%) than IL (38.3±3.2% t4=3.60, p=0.02). E Diagrams of coronal slices showing the injection sites (yellow dots) where ChR2-expressing AAV2 was injected in the PrL of 5 anesthetized rats (left), and light (DIC, middle) and fluorescent (YFP, right) images showing the expression site of ChR2 3 weeks after intra-PrL injection of ChR2-AAV2. F Same experimental setup as shown in “E” for intra-IL injection of ChR2. G, H Confocal images showing ChR2-expressing IL (G) or PrL (H) neurons from rats with intra-IL or intra-PrL expression of ChR2, respectively. Inset, action potentials elicited by optogenetic stimulation. I, J Diagrams and confocal images showing ChR2-expressing neural fibers in the NAcSh (I) and NAcCo (J) from rats with intra-IL or intra-PrL expression of ChR2. K Example traces (left) and summarized results (right) showing that optogenetic stimulation (1 ms) of ChR2-expressing fibers in NAc slice from a rat with intra-IL or intra-PrL expression of ChR2 elicited synaptic currents at −70 mV in a NAcSh MSN that were inhibited by the AMPAR-selective antagonist NBQX (relative current in NBQX: IL-to-NAcSh, 7.1±1.8%; t4=50.6, p<0.01; PrL-to-NAcCo, 5.6±0.9%; t4=102.1, p<0.01). L Example traces showing that currents from IL-to-NAcSh (left) or PrL-to-NAcCo (right) synapses exhibited short delays after presynaptic stimulation. M Examples traces showing that currents from the IL-to-NAcSh (left) or PrL-to-NAcCo (right) synapses by optogenetic stimuli (1-ms stimulation duration) were prevented by TTX (1 μM). N Example traces (left), trials (middle) of these traces, and summarized results (right) showing that the success rate of synaptic responses was decreased by TTX at both IL-to-NAcSh (t5=10.5, p<0.01, paired t-test) and PrL-to-NAcCo (t6=7.6, p<0.01, paired t-test) synapses. O Example traces (left), trials of these traces (middle), and summarized results (right) showing that reducing the bath concentration of Ca2+ (from 2.5 to 1.0mM) reduced the success rate of IL-to-NAcSh (t =4.3, p=0.01, paired t-test) and PrL-to-NAcCo (t4=8.5, p<0.01, paired t-test) synapses. P Example EPSCs (upper) and summarized results (lower) showing that optogenetic stimulation of the PrL projection elicited substantially larger responses in NAcCo neurons, whereas stimulation of the IL projection elicited substantially larger responses in NAcSh neurons (F1,65=60.0, p<0.01, two-way ANOVA; p<0.01, NAcCo vs. NAcSh for either PrL or IL, Bonferroni posttest).*, p<0.05; **, p<0.01.