Abstract
Background
Cardiac resynchronization therapy (CRT) is one of the most exciting recent advancements in heart failure (HF) treatment.
Methods
This review surveys the available literature regarding the effectiveness of CRT in treating patients with HF.
Results
By targeting ventricular dyssynchrony, CRT attempts to give the failing heart a mechanical advantage that can substantially improve both symptoms and mortality.
Conclusion
CRT results in short-term and long-term improvement in cardiac structure and function, often leading to enhanced quality of life and, for some patients, enhanced survival.
Keywords: Cardiac electrophysiology, cardiac resynchronization therapy, heart failure, myocardial infarction
INTRODUCTION
Cardiac resynchronization therapy (CRT) is one of the most exciting recent advancements in heart failure (HF) treatment. By targeting ventricular dyssynchrony, a condition that plagues as many as one-third of patients with highly symptomatic systolic HF,1 CRT attempts to give the failing heart a mechanical advantage that can substantially improve symptoms and mortality. This review covers the pathophysiology of cardiac dyssynchrony, the potential benefits of successful resynchronization, and current and future directions for this therapy.
CARDIAC DYSSYNCHRONY
Electrical Dyssynchrony
Under normal conditions, the myocardium is activated by a uniform, high-velocity electrical waveform that propagates through the His-Purkinje system, resulting in synchronized depolarization of the ventricles. In diseased hearts, altered electrochemical substrate and impaired conduction fibers can change the velocity and uniformity of electrical propagation, resulting in areas of activation delay. If the delay is significant enough, it manifests as lengthening of the QRS complex on the surface 12-lead electrocardiogram (ECG). Because the QRS complex represents the summation vector of electrical forces generated by the ventricular myocardium during the course of ventricular systole, a prolonged QRS segment suggests impaired conduction velocity and its product, electrical dyssynchrony (Figure 1).
Figure 1.
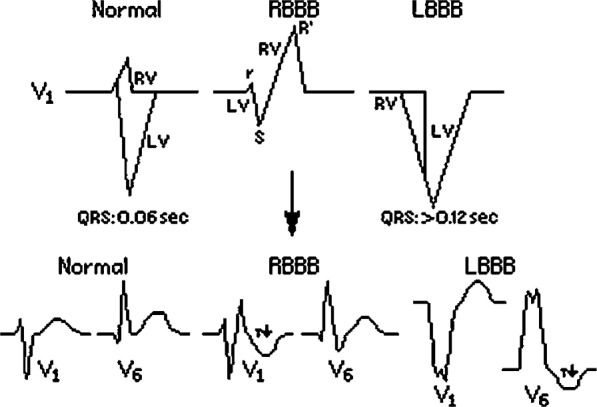
The difference between the normal narrow QRS and wide QRS as seen in left bundle branch block (LBBB) and right bundle branch block (RBBB). Also note the relative contribution of the left ventricle (LV) and right ventricle (RV) to the QRS complex. (Source: University of San Francisco, http://www.usfca.edu/fac-staff/ritter/section.htm. Reprinted with permission.)
A direct relationship exists between QRS duration and depressed left ventricular ejection fraction (LVEF).2 In addition, QRS duration correlates with worsening symptoms: while the prevalence of a prolonged QRS (>120 ms) is approximately 20% in the general HF population, it is approximately 35% among patients with more symptomatic HF.3
Mechanical Dyssynchrony
Mechanical dyssynchrony can be the physical manifestation of electrical dyssynchrony. There are 3 types of mechanical dyssynchrony:
-
1.
Intraventricular dyssynchrony within the left ventricle that often is most prominent in patients with left bundle branch block (LBBB) because of a delay between the relatively early-activated interventricular septum and late-activated posterolateral wall
-
2.
Interventricular dyssynchrony between the left and right ventricles that is most often the result of delayed activation of the left ventricle because of LBBB
-
3.
Atrioventricular (AV) dyssynchrony secondary to prolonged or absent AV nodal conduction, potentially coupled with His-Purkinje system dysfunction
Any form of mechanical dyssynchrony can prolong the periods of isovolumic contraction and isovolumic relaxation (during which no movement of blood occurs) and consequently decrease cardiac pumping efficiency. Additionally, a dyssynchronous dilated left ventricle can result in mitral regurgitation (MR) because of lack of leaflet coaptation and papillary muscle dysfunction.4
While a prolonged QRS is the best marker for dyssynchrony, some evidence suggests that mechanical dyssynchrony can manifest in the absence of QRS prolongation.5 Because QRS morphology and duration are influenced only by significant myocardial masses, regional discrepancies represented by small vectors are often masked. In a damaged myocardium (eg, in patients who have suffered myocardial infarction), areas of impaired contractility can produce mechanical dyssynchrony without any detectable electrical conduction disturbance.
Cardiac Remodeling
One consequence of long-standing cardiac dyssychrony is a pathologic process known as remodeling. Cardiac remodeling manifests clinically as left ventricle dilatation, worsening systolic and diastolic function, and progressive HF.
CRT
Procedure Details
CRT is typically accomplished by adding a left ventricular (LV) pacing lead to a standard pacemaker or defibrillator system that generally includes only a right ventricular (RV) lead and possibly a right atrial lead (Figure 2). The RV lead most often rests in the apex of the right ventricle. The LV lead is placed through the coronary sinus onto the lateral or posterolateral wall of the left ventricle. When the 2 leads are activated, coordinated pacing of the left ventricle and right ventricle results.6
Figure 2.
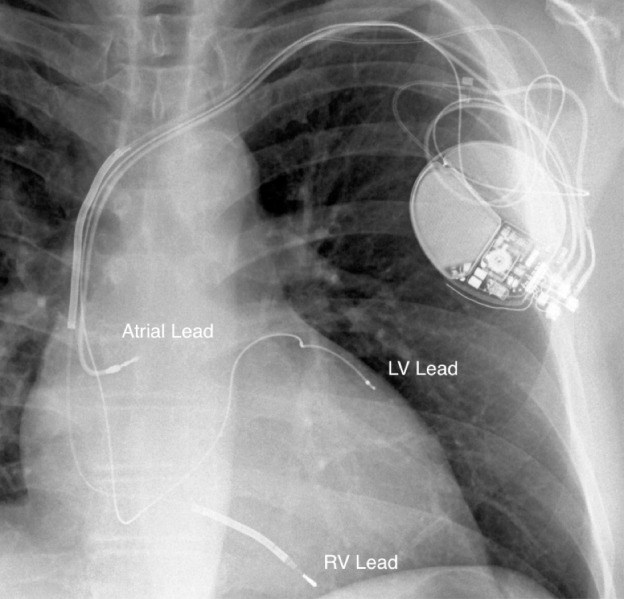
X-ray of a cardiac resynchronization therapy system showing lead positioning. LV, left ventricular; RV, right ventricular.
Patient Selection
At present, patients are eligible for CRT based on QRS duration >120 ms, LVEF ≤35%, and at least moderate symptoms of chronic HF despite treatment with optimal medical therapy. These criteria are based on the enrollment criteria of the landmark CRT trials. Although these cutoffs were arbitrarily chosen, studies that have tried to expand these criteria either do not exist or have not had much success.
Multiple large studies have utilized echocardiographic criteria designed to assess mechanical dyssynchrony to evaluate the efficacy of CRT in patients with LV dyssychrony but relatively narrow QRS duration (≤130 ms). Universally, these trials have failed to demonstrate any morbidity or mortality advantage in this population when treated with CRT.7,8 Moreover, the EchoCRT (Echocardiography Guided Cardiac Resynchronization Therapy) study even showed an increase in mortality with CRT implantation in this cohort.9 As a result, a prolonged QRS duration is currently the only validated marker of dyssynchrony that qualifies patients for CRT.
The threshold of LVEF ≤35% has been well established through large clinical trials. Data indicating that CRT is beneficial in patients with a higher LVEF are currently lacking.
A notable exception to the above selection criteria is a case in which a patient is expected to require frequent cardiac pacing as a result of AV block. Consistent pacing with a single RV lead can create the same type of dyssychrony that CRT is meant to correct, making it advantageous to implant a CRT device in these patients.10 To test this hypothesis, the BLOCK HF (Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block) trial compared CRT to RV pacing in patients with an LVEF ≤50% in patients expected to pace frequently.11 The results confirmed that CRT significantly reduced the combined endpoint of mortality, HF-related urgent care visits, and increased left ventricle end-systolic diameter (a marker of LV dysfunction) by 26% compared to RV pacing alone. Thus, in patients who are intended for device implantation to treat frequent AV block, CRT may be considered as a means of preventing pacing-induced ventricular dysfunction.
Physiologic Benefits
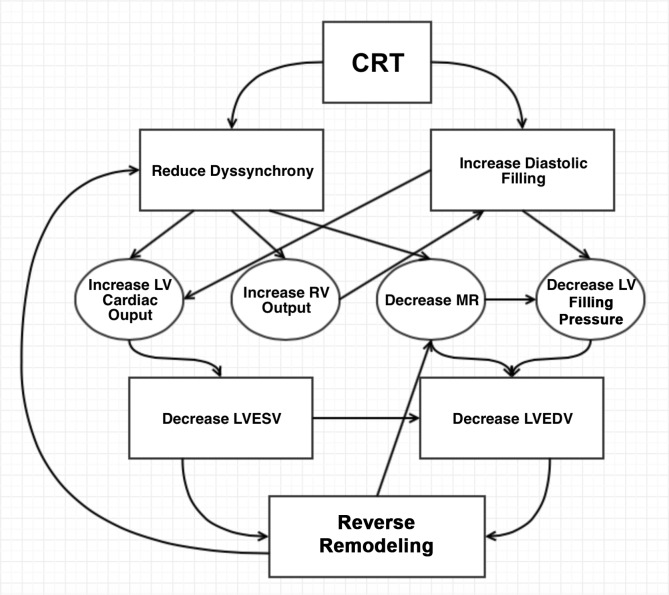
As soon as it is activated, CRT can reduce all 3 types of cardiac mechanical dyssynchrony.12,13 Coordinated contraction of the ventricles gives the previously dyssynchronous heart an instant mechanical advantage that augments cardiac output. In addition to the immediate hemodynamic benefits, during a span of months, CRT leads to further improvement in the structure and function of the heart (Figure 3). These long-term changes are known collectively as reverse remodeling that is usually quantified by a reduction in LV size and an improvement in LV function. Reverse remodeling is a consistent finding in CRT-treated patients with baseline symptomatic HF and long QRS duration.14,15 Moreover, Yu et al showed that this benefit disappears following CRT withdrawal.16
Figure 3.
Proposed mechanisms of benefit of biventricular pacing. Cardiac resynchronization therapy (CRT) leads to improved intraventricular and interventricular synchrony, as well as an increase in diastolic filling time. These changes improve both systolic and diastolic function of the heart that in turn leads to a decrease in systolic and diastolic left ventricle (LV) volume. Over time, these hemodynamic changes lead to long-standing improvements known as reverse remodeling. Reverse remodeling will additionally improve cardiac synchrony and decrease secondary mitral regurgitation (MR), forming a positive feedback loop. LA, left atrial; LVEDV, left ventricle end-diastolic volume; LVESV, left ventricle end-systolic volume; RV, right ventricle.
The degree of reverse remodeling seen with CRT is similar to that seen following treatment with angiotensin-converting enzyme inhibitors and beta blockers, beneficial changes that have been linked to reduced morbidity and mortality in all classes of systolic HF. Reverse remodeling is thought to be one reason for the observed improved clinical status and decreased mortality reported in many CRT trials.12 The resultant geometric changes may also lead to less dilatation of the mitral annulus and thereby to a decrease in the severity of MR, a common comorbid condition in patients with HF.
At the cellular level, CRT improves sarcomere shortening via increased peak calcium levels that improve cardiac contractility and systolic function. CRT also enhances beta-adrenergic responsiveness by upregulating the number of beta receptors on myocardial cell surfaces. The increase in the number of beta receptors is particularly important because myocytes in failing hearts generally have reduced adrenergic responsiveness, leading to higher circulating catecholamine levels that can lead to accelerated HF. In an animal model of dyssynchronous HF, CRT decreased myocardial catecholamine to almost normal levels.17 CRT can also increase mitochondrial adenosine triphosphate synthase activity by reversing mitochondrial oxidative posttranslational modification. This enzymatic upregulation leads to improved myocyte function and more efficient energy metabolism.18
Clinical Benefits
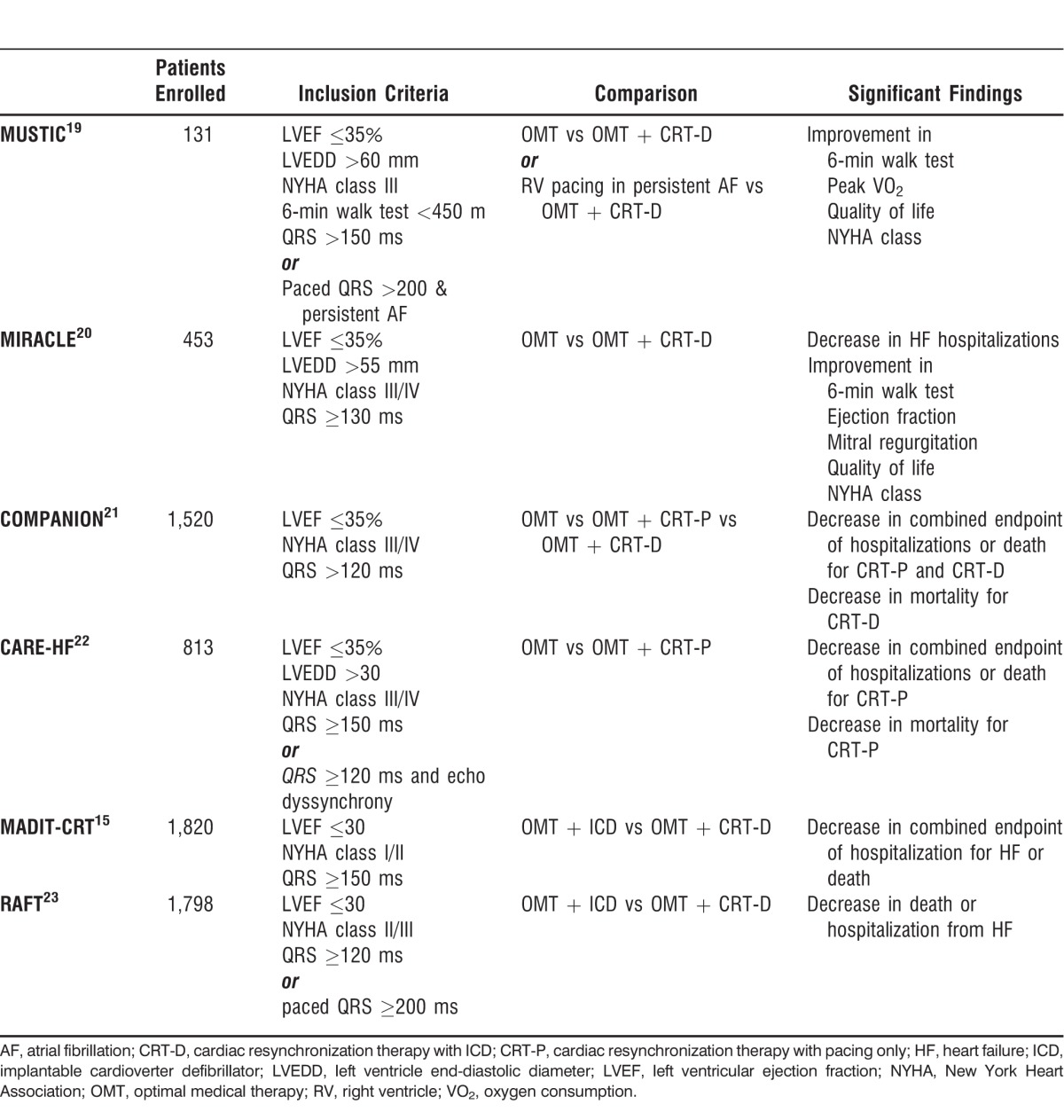
Multiple large randomized trials have shown dramatic clinical benefits of CRT in selected HF populations. These trials are summarized below and in the Table.
Table.
Summary of Major Randomized Cardiac Resynchronization Therapy Trials
Multisite Stimulation in Cardiomyopathy (MUSTIC) Trial
The MUSTIC trial, published in 2001, was the first large trial demonstrating the clinical benefits of CRT.19 It was a single-blind crossover study enlisting patients with New York Heart Association (NYHA) class III HF, LVEF ≤35%, left ventricle end-diastolic diameter >60 mm, and QRS duration >150 ms. The MUSTIC investigators compared exercise tolerance and quality of life during active biventricular pacing for 3 months to exercise tolerance and quality of life during right ventricle–only backup pacing for a separate 3 months. The trial showed a statistically significant improvement in 6-minute walk distance (the primary endpoint), as well as improved quality of life and peak oxygen consumption following CRT. Because of the short duration of the study and crossover design, the study was not able to show a mortality difference. Nevertheless, the MUSTIC trial was important because it was the first trial demonstrating clinical improvement with CRT and paved the way for later randomized controlled trials.
Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Trial
Reported in 2002, the MIRACLE trial randomized 453 patients in sinus rhythm with NYHA class III-IV HF, LVEF ≤35%, and QRS duration ≥130 ms to receive biventricular pacing or no pacing.20 During the 6-month follow-up, CRT showed a decrease in hospitalizations because of HF as well as significant improvements in 6-minute walk distance, NYHA class, and quality of life score. A significant improvement in walking distance was noticed in the CRT group in as early as 3 months. In addition, the MIRACLE trial showed that resynchronization therapy is an effective adjunct to pharmacologic therapy in reducing the secondary combined endpoint of HF hospitalization or death. Despite these positive results, the MIRACLE trial was not sufficiently powered to detect an improvement in mortality with CRT.
Comparison of Medical Therapy, Pacing, and Defibrillation in HF (COMPANION) Trial
The COMPANION trial included 1,520 patients and was the first trial able to detect improvement in the primary combined endpoint of hospitalization or death from any cause.21 The trial enrolled patients with NYHA class III-IV HF, LVEF ≤35%, and QRS duration >120 ms. The trial had 3 treatment arms: patients were randomly assigned to optimal medical therapy (OMT) alone, OMT plus CRT with pacing only (CRT-P), or OMT plus CRT with a defibrillator (CRT-D). At 1 year, the CRT-D group, but not the CRT-P group, had a significant reduction in overall mortality compared to the group receiving OMT alone. The CRT-P group barely missed statistical significance for overall mortality (P=0.059). The exciting results of the COMPANION study showed that CRT-D had a definite mortality benefit and suggested that CRT may improve mortality, even in the absence of a defibrillator.
Cardiac Resynchronization-HF (CARE-HF) Trial
In 2005, the CARE-HF trial attempted to clarify the mortality benefit of CRT independent of the mortality benefit of defibrillation.22 This trial enrolled 813 patients with NYHA class III-IV HF, QRS duration ≥120 ms, echocardiographic dyssynchrony, and LVEF ≤35%. Of note, the vast majority of CARE-HF subjects had very long QRS duration (median 160 ms). Only approximately 8% of patients had QRS duration between 120-150 ms, and enrollment of these patients required mechanical dyssynchrony as determined by echocardiography. The primary endpoint was a composite of all-cause mortality or hospitalization for a major cardiovascular event, and the secondary endpoint was all-cause mortality. Compared to OMT alone, CRT-P was associated with a significant (26%) reduction in all-cause mortality and hospitalization for major cardiovascular events at 29 months. Most important, CARE-HF was the first trial to show definitively that CRT-P had a mortality benefit, even in the absence of implantable cardioverter defibrillator (ICD) therapy.
Multicenter Automatic Defibrillator Implantation with Cardiac Resynchronization Therapy (MADIT-CRT) Trial
In 2009, the MADIT-CRT trial randomized 1,820 patients with LVEF ≤30% and QRS duration ≥150 ms to biventricular pacing or no-pacing groups.15 Significantly, the MADIT-CRT trial enrolled patients with mild (NYHA class I-II) HF, a group that had previously been excluded from CRT trials. This study showed that CRT produced a 29% reduction in the primary combined endpoint of HF events (defined by a need for intravenous diuretics) and mortality. However, further analysis showed that this reduction was derived almost exclusively from HF events, slightly weakening the impact of the study. Nonetheless, MADIT-CRT demonstrated that even mildly symptomatic HF patients may benefit from CRT.
Resynchronization-Defibrillation for Ambulatory HF (RAFT) Trial
The most recent large randomized CRT trial, RAFT, followed in the footsteps of CARE-HF to delineate the mortality advantage conferred by CRT independent of defibrillation.23 The trial randomized 1,798 patients with NYHA class II-III HF, LVEF ≤30%, and QRS duration ≥120 ms to receive either an ICD alone or an ICD plus CRT. The study recorded that CRT produced a 7% reduction in the primary outcome of death from any cause or hospitalization for HF. RAFT further confirmed the benefits of CRT over and above the benefits of defibrillator therapy alone.
An important overriding theme in the major CRT trials has been the evolution of patient selection criteria. Initial trials predominantly enrolled patients at a later stage of disease who had severe LV dysfunction, highly symptomatic HF, and very long QRS durations. More recent trials have expanded inclusion criteria to patients with more compensated HF and shorter QRS durations. Future trials will probably attempt to continue this trend, focusing on patients earlier in the course of disease progression. However, as current and future trials enroll healthier patients, it may become increasingly difficult to show significant benefit. This difficulty will be one of the challenges for CRT therapy research in the years to come.
Current Guidelines
In 2012, the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society updated their guidelines for CRT.24 The major changes to the guidelines involved expanding the pool of patients eligible for CRT to include patients with asymptomatic or mildly symptomatic HF (NYHA class I-II).
CRT NONRESPONDERS
As CRT was more widely implemented, it became clear that even among patients with prolonged QRS intervals, approximately 30% of patients (and as high as 40%-50% in some studies) do not achieve the expected clinical benefits.25 The etiology of CRT failure to help some patients is an area of active investigation. The following theories have been proposed to explain CRT nonresponse.
Inconsistent Pacing
Biventricular pacing is maximally beneficial when the ventricle is paced very frequently (as close to 100% of the time as possible).26 If the patient's own heart rate exceeds the device's programmed rate, pacing is inhibited and the potential benefits of resynchronization are missed completely. Thus, the simplest explanation for lack of improvement with CRT is inconsistent pacing.
Lack of Mechanical Dyssynchrony
Multiple studies have demonstrated that ventricular dyssynchrony is the major pathological derangement associated with mechanical inefficiency and deleterious biological effects that are improved by CRT.27,28 It has been shown that the presence of baseline mechanical dyssychrony was associated with a response to resynchronization therapy.29 However, no potential marker of mechanical dyssynchrony reliably predicts response. The lack of predictability is especially evident when different investigative centers attempt to replicate each other's work: test performance varies widely between centers.30
Myocardial Scar
Scar burden in patients with ischemic cardiomyopathy may be an important determinant of response to CRT. A large myocardial scar burden––assessed by either single-photon emission computed tomography imaging or magnetic resonance imaging (MRI)––is associated with a poor response to CRT and a poor prognosis.31-33 The association between higher scar burden and poor CRT response may partially explain why patients with nonischemic cardiomyopathy tend to respond better to CRT than those with ischemic cardiomyopathy.
The location of scarring in reference to the LV lead has been shown to be an important factor in the success of CRT.34 A lead with its tip placed in a scar region is unlikely to pace the left ventricle effectively, if at all.
Lead Position
Ideally, the LV lead of a CRT device would be placed at the precise location of latest electromechanical activation. Theoretically, this positioning would result in optimal resynchronization. However, in practice, positioning the LV lead transvenously is limited by the accessibility of suitable epicardial coronary veins. Based on population-based LV activation mapping, the usual target for CRT lead placement is the lateral or posterolateral left ventricle.35
When coronary sinus anatomy is unfavorable for optimal LV lead placement, operative placement of an epicardial lead allows more flexibility, with the obvious caveat that surgery is required (with its attendant risks) and recovery time is longer. It is important to note that both procedural and surgical placement of the LV lead results in epicardial activation of the left ventricle rather than endocardial activation that may be more physiologic.
REDUCING THE RATE OF CRT NONRESPONSE
The diversity of mechanisms that reduce CRT responsiveness makes it difficult to correlate nonresponse with any single clinical, demographic, or electrocardiographic finding prior to implantation. Thus, a multifaceted approach is needed to improve the CRT response rate.
Improved Patient Selection
Subgroup analysis of major randomized CRT studies demonstrates that a longer QRS duration is associated with a greater effect of CRT therapy. In some trials, patients with QRS duration <150 ms did not benefit from CRT at all.36 Interestingly, the extent of QRS shortening postimplantation is highly correlated with a response to CRT.37
Recently, there has been considerable interest in utilizing noninvasive imaging modalities to more accurately assess LV dyssynchrony and to predict response to CRT. Multiple small studies using imaging-derived markers of dyssynchrony have demonstrated varying degrees of success in predicting a response to CRT, leading to the large, multicenter Predictors of Response to CRT (PROSPECT) study that examined 12 echocardiographic parameters of dyssynchrony and correlated the degree of dyssynchrony with response to CRT.38 The ability of these parameters to predict response to CRT varied widely, with unacceptably low interobserver reproducibility and minimal correlation between mechanical dyssynchrony and response to CRT.39
Cardiac MRI is emerging as a promising imaging modality for more standardized detection and grading of LV dyssynchrony. Moreover, MRI offers the unique opportunity to assess both dyssynchrony and scar extent in a single session.35 Small studies have shown promising concordance between MRI-assessed scar burden, markers of dyssynchrony, and CRT response.40,41 However, at present, large trials prospectively assessing whether cardiac MRI can be used to predict responsiveness to CRT are lacking.
No imaging parameter or combination of imaging parameters has been shown to predict a favorable response to CRT, which continues to limit the use of imaging criteria in practice. However, as technology improves and advanced imaging modalities become more commonplace, there may be opportunities to discover more reliable and standardized markers of mechanical dyssynchrony.
Optimal LV Lead Positioning
One factor in reducing the rate of CRT nonresponders is optimal LV lead placement. Typically, the LV lead is placed in the lateral or posterolateral branches of the coronary sinus, as most evidence points to these areas as advantageous.
One study demonstrated that LV leads positioned near the site of latest mechanical activation (as assessed by two-dimensional echocardiographic speckle tracking strain assessment) led to a more favorable response to CRT compared to leads placed near sites of earlier mechanical activation.30 In addition, post hoc analysis of LV lead positioning in the MADIT-CRT cohort revealed that apical positioning, compared to basal positioning, of the LV lead resulted in a significant increase in HF events and death.42 The MADIT-CRT investigators therefore recommend that apical positioning of the LV lead should be avoided.
Preliminary data also show that endocardial pacing (ie, within the left ventricle cavity) may lead to improved response rates in CRT. Endocardial pacing acutely improves LV contractile function compared to epicardial pacing.43,44 The primary results of the Alternate Site Cardiac Resynchronization Therapy (ALSYNC) trial demonstrated dramatic clinical improvement with endocardial placement of an LV lead. Patients in whom conventional CRT implantation failed or who were nonresponders had an endocardial lead placed via a trans–atrial-septal approach. More than half of these patients had significant improvement in symptoms and LVEF.45 One considerable caution pertaining to left-sided endocardial pacing, however, is the potential for thrombus formation and therefore risk for stroke and systemic embolism.
Fully intracardiac leadless pacemakers may represent the future of pacing and CRT. These tiny devices could be powered either by self-contained batteries or energy transmitted acoustically or magnetically. The enhanced flexibility inherent in such a device could allow more strategic endocardial pacing and perhaps a better response to CRT.46,47
Rate and Rhythm Control
As detailed above, the response to CRT is maximized when the ventricle is paced as close to 100% of the time as possible. Normally functioning CRT devices will not pace above their programmed upper rate limit. Thus, in patients prone to relative tachycardia, pacing may be inhibited, negating the potential benefits of CRT. For patients with atrial fibrillation (AF) with an uncontrolled ventricular response, pharmacologic rate or rhythm control can be effective. If medical therapy fails, AF ablation or AV nodal ablation may be considered. Tachycardia because of atrial flutter or other supraventricular tachycardia can be managed similarly with pharmacologic, ablation-based rate-control, or rhythm-control strategies.
Postimplantation Clinical Optimization and Device Optimization
Merely implanting a CRT device may not be sufficient to ensure an optimal response. A multidisciplinary approach including tailored medical management of HF and device settings may be able to reduce adverse outcomes. For example, a study published in 2009 documented a protocol-driven approach for CRT optimization involving an HF physician, electrophysiologist, and focused echocardiography.48 This study led to changes in device settings and/or other therapy modifications in 74% of patients. These changes resulted in significantly fewer adverse events compared with standard non–protocol-driven programming. Mullens et al sought to evaluate the benefit of protocol-driven HF optimization after CRT implantation. While this intervention did not reduce mortality, it was associated with fewer adverse events compared with standard programming.49 In addition, it is important to continue optimization of medical therapy following CRT implantation. Medical therapy not only directly treats congestive HF, but it can also improve control of the intrinsic heart rate, improving the percentage of CRT pacing. Thus, among the population that qualifies for CRT, outcomes depend upon more than the presence of a resynchronization device.
Some devices, both market-released and experimental, are able to optimize their own programming. For example, the AdaptivCRT algorithm (Medtronic Inc.) self-regulates the AV interval and interventricular pacing delay in response to whether AV conduction is present and intact and in response to native interventricular timing.50 Other manufacturers have similar algorithms. In addition, an automatic device optimization experiment using sensors in the tip of the atrial pacing lead is currently ongoing (RESPOND CRT). This system would automatically adjust AV delay and interventricular delay based on self-recorded feedback. The goal of the RESPOND CRT trial is to compare this novel optimization strategy against echocardiography-based device optimization.51
CURRENT CONTROVERSY AND FUTURE DIRECTIONS
The future of CRT involves research directed at expanding the pool of patients who may derive benefit from this technology. In an attempt to improve patient selection, the eligibility criteria in recent investigations have been expanded to include 4 subgroups previously excluded from many randomized controlled trials: patients with AF, patients with a relatively narrow QRS duration (<130 ms), patients with right bundle branch block (RBBB), and patients with HF with preserved ejection fraction (EF).
AF
Despite the high prevalence of AF in patients with HF, most clinical trials examining CRT have excluded this group. Most of the data regarding this group of patients come from a metaanalysis of prospective cohort studies comparing the impact of CRT on 1,164 patients in the AF group versus 1,323 patients in the sinus rhythm group.52 This metaanalysis showed that patients with AF had greater improvement in EF but smaller improvements in functional outcomes than those in sinus rhythm. Mortality was similar between the groups.
A CRT device will only pace if the patient's intrinsic rate is lower than the programmed rate. In patients with AF who are prone to rapid ventricular response, their tachycardia may limit the percentage of paced beats. Thus, to achieve maximal benefit in this patient population, AV nodal conduction must be controlled to maintain a normal ventricular rate. AV nodal conduction can be slowed medically with negative chronotropic medications such as beta blockers, calcium channel blockers, and/or digoxin. If medical therapy is inadequate, catheter ablation of the AV node results in complete AV block and therefore 100% pacing.53
RBBB
The preponderance of data in randomized controlled clinical trials evaluating the utility of CRT in HF was obtained from patients with LBBB. Patients with RBBB or nonspecific intraventricular conduction delay (IVCD) were underrepresented in the tested populations.
Pure proximal RBBB, the most common type of RBBB, does not disrupt normal LV activation.54 Thus, it is intuitively unclear how the addition of an LV lead could improve synchrony in patients whose RV activation is delayed. A possible explanation of CRT utility in RBBB may be that complete RBBB can mask underlying concomitant delay in the left bundle branch.55
Post hoc analysis of QRS morphology in the MADIT-CRT trial demonstrated no clinical benefit in patients with a non-LBBB QRS pattern (ie, RBBB or IVCD). Patients with LBBB had a significant decrease in the combined endpoint of mortality and HF admissions, as well as a reduction in the number of ventricular tachyarrhythmias. Patients with non-LBBB QRS patterns experienced none of these benefits and in fact demonstrated a nonsignificant trend toward higher mortality when treated with CRT-D. Interestingly, for patients with nonspecific IVCD, the more their QRS morphology resembled LBBB, the better their outcomes.56 The reasons for the potentially disparate efficacy between LBBB and non-LBBB morphology patient groups is unclear, but a possibility is the baseline higher event rate seen in the LBBB population (making detection of benefit easier to accomplish). As similar physical benefits (ie, echocardiographic improvements) were seen among non-LBBB patients, the larger benefit seen in those with LBBB cannot be explained solely by reverse mechanical remodeling. Perhaps the electrical effects of CRT are more to credit for LBBB patients' improved response. Further investigation will be required to answer these questions more definitively.
A closer look at the results of the REVERSE,14 MADIT-CRT,15 and RAFT23 trials demonstrates a striking improvement in CRT outcomes for patients with QRS duration ≥150 ms versus those with QRS duration <150 ms. This difference in outcomes is especially interesting because a recent electrical mapping study showed that true LBBB was only seen when a QRS duration >140 ms was present.57 This finding may also help explain the lack of benefit for CRT in patients with QRS duration <130 ms, as shown in the RethinQ,7 ESTEEM-CRT,8 and EchoCRT9 trials.
The updated CRT guidelines now differentiate between LBBB and non-LBBB morphologies with regard to QRS duration cutoffs. Guidelines support the use of CRT more strongly in the presence of RBBB only when QRS duration is >150 ms, rather than the lower cutoff of >120 ms when LBBB is present.
HF With Preserved EF
Because data regarding the efficacy of CRT in patients with HF and LVEF >35% are lacking, current guidelines limit CRT therapy to those with LVEF ≤35%. However, QRS prolongation is a risk factor for all-cause mortality independent of the degree of HF, and the risk associated with QRS prolongation may be similar regardless of EF.58
A 2010 post hoc analysis of the PROSPECT trial attempted to directly explore the potential benefits of CRT for patients with LVEF >35%. Those investigators found that despite the study's stated inclusion criterion of LVEF ≤35%, when subjects' echocardiograms were analyzed in a core laboratory, 24% of patients actually had LVEF >35%.59 Even though this higher LVEF group had lower LV volumes, shorter QRS durations, and more frequent ischemic cardiomyopathy (which generally responds less favorably to CRT), outcomes were not different compared to the group with LVEF ≤35%. The 2 groups had similar reductions in left ventricle end-systolic volumes. Thus, patients with less severely reduced LVEF may benefit from CRT.
The currently ongoing MIRACLE-EF trial is a multinational randomized investigation enrolling more than 2,000 patients with LVEF 36%-50%, LBBB with QRS ≥130 ms, and NYHA class II-III symptoms. The results of this trial should help answer the question of whether CRT is beneficial in HF patients with only mildly reduced left ventricle systolic function.
CONCLUSION
CRT is an effective treatment for selected patients with chronic HF and a prolonged QRS interval. It is one of the most powerful recent advances for reducing morbidity and mortality in the challenging HF population. CRT results in both short-term and long-term improvement in cardiac structure and function, leading to enhanced quality of life and, for some populations, enhanced survival. Ongoing and future research will continue to investigate ways to reduce the frequency of nonresponse to CRT while seeking to expand the pool of patients eligible for this life-saving therapy.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.McAlister FA, Ezekowitz J, Hooton N, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA. 2007 Jun 13;297(22):2502–2514. doi: 10.1001/jama.297.22.2502. [DOI] [PubMed] [Google Scholar]
- 2.Shenkman HJ, Pampati V, Khandelwal AK, et al. Congestive heart failure and QRS duration: establishing prognosis study. Chest. 2002 Aug;122(2):528–534. doi: 10.1378/chest.122.2.528. [DOI] [PubMed] [Google Scholar]
- 3.Silvet H, Amin J, Padmanabhan S, Pai RG. Prognostic implications of increased QRS duration in patients with moderate and severe left ventricular systolic dysfunction. Am J Cardiol. 2001 Jul 15;88(2):182–185. doi: 10.1016/s0002-9149(01)01619-8. A6. [DOI] [PubMed] [Google Scholar]
- 4.Ypenburg C, Lancellotti P, Tops LF, et al. Mechanism of improvement in mitral regurgitation after cardiac resynchronization therapy. Eur Heart J. 2008 Mar;29(6):757–765. doi: 10.1093/eurheartj/ehn063. [DOI] [PubMed] [Google Scholar]
- 5.Hawkins NM, Petrie MC, MacDonald MR, Hogg KJ, McMurray JJ. Selecting patients for cardiac resynchronization therapy: electrical or mechanical dyssynchrony? Eur Heart J. 2006 Jun;27(11):1270–1281. doi: 10.1093/eurheartj/ehi826. [DOI] [PubMed] [Google Scholar]
- 6.Beshai JF, Khunnawat C, Lin AC. Mechanical dyssynchrony from the perspective of a cardiac electrophysiologist. Curr Opin Cardiol. 2008 Sep;23(5):447–451. doi: 10.1097/HCO.0b013e32830a95f1. [DOI] [PubMed] [Google Scholar]
- 7.Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007 Dec 13;357(24):2461–2471. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]
- 8.Donahue T, Niazi I, Leon A, Stucky M, Herrmann K, Investigators ESTEEM-CRT. Acute and chronic response to CRT in narrow QRS patients. J Cardiovasc Transl Res. 2012 Apr;5(2):232–241. doi: 10.1007/s12265-011-9338-3. [DOI] [PubMed] [Google Scholar]
- 9.Ruschitzka F, Abraham WT, Singh JP, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013 Oct 10;369(15):1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 10.Wilkoff BL, Kudenchuk PJ, Buxton AE, et al. The DAVID (Dual Chamber and VVI Implantable Defibrillator) II trial. J Am Coll Cardiol. 2009 Mar 10;53(10):872–880. doi: 10.1016/j.jacc.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 11.Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013 Apr 25;368(17):1585–1593. doi: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]
- 12.Leclercq C, Bleeker GB, Linde C, et al. Cardiac resynchronization therapy: clinical results and evolution of candidate selection. Eur Heart J Suppl. 2007 Dec;9(Suppl I):I94–I106. [Google Scholar]
- 13.Gras D, Leclercq C, Tang AS, Bucknall C, Luttikhuis HO, Kirstein-Pedersen A. Cardiac resynchronization therapy in advanced heart failure: the multicenter InSync clinical study. Eur J Heart Fail. 2002 Jun;4(3):311–320. doi: 10.1016/s1388-9842(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 14.Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008 Dec 2;52(23):1834–1843. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009 Oct 1;361(14):1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 16.Yu CM, Chau E, Sanderson JE, et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation. 2002 Jan 29;105(4):438–445. doi: 10.1161/hc0402.102623. [DOI] [PubMed] [Google Scholar]
- 17.Chakir K, Daya SK, Aiba T, et al. Mechanisms of enhanced beta-adrenergic reserve from cardiac resynchronization therapy. Circulation. 2009 Mar 10;119(9):1231–1240. doi: 10.1161/CIRCULATIONAHA.108.774752. Erratum in: Circulation. 2009 May 19;119(19):e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweier JL, Chen CA, Talukder MA. Cardiac resynchronization therapy and reverse molecular remodeling: importance of mitochondrial redox signaling. Circ Res. 2011 Sep 16;109(7):716–719. doi: 10.1161/CIRCRESAHA.111.253864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001 Mar 22;344(12):873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 20.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002 Jun 13;346(24):1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 21.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004 May 20;350(21):2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 22.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005 Apr 14;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 23.Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010 Dec 16;363(25):2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 24.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2012 2013 Jan 22;61(3):e6–e75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Birnie DH, Tang AS. The problem of non-response to cardiac resynchronization therapy. Curr Opin Cardiol. 2006 Jan;21(1):20–26. doi: 10.1097/01.hco.0000198983.93755.99. [DOI] [PubMed] [Google Scholar]
- 26.Koplan BA, Kaplan AJ, Weiner S, Jones PW, Seth M, Christman SA. Heart failure decompensation and all-cause mortality in relation to percent biventricular pacing in patients with heart failure: is a goal of 100% biventricular pacing necessary? J Am Coll Cardiol. 2009 Jan 27;53(4):355–360. doi: 10.1016/j.jacc.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 27.Gorcsan J, 3rd, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting--a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008 Mar;21(3):191–213. doi: 10.1016/j.echo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Kass DA. Pathobiology of cardiac dyssynchrony and resynchronization. Heart Rhythm. 2009 Nov;6(11):1660–1665. doi: 10.1016/j.hrthm.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bleeker GB, Mollema SA, Holman ER, et al. Left ventricular resynchronization is mandatory for response to cardiac resynchronization therapy: analysis in patients with echocardiographic evidence of left ventricular dyssynchrony at baseline. Circulation. 2007 Sep 25;116(13):1440–1448. doi: 10.1161/CIRCULATIONAHA.106.677005. [DOI] [PubMed] [Google Scholar]
- 30.Delgado V, van Bommel RJ, Bertini M, et al. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation. 2011 Jan 4;123(1):70–78. doi: 10.1161/CIRCULATIONAHA.110.945345. [DOI] [PubMed] [Google Scholar]
- 31.Adelstein EC, Tanaka H, Soman P, et al. Impact of scar burden by single-photon emission computed tomography myocardial perfusion imaging on patient outcomes following cardiac resynchronization therapy. Eur Heart J. 2011 Jan;32(1):93–103. doi: 10.1093/eurheartj/ehq389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. 2007 Jan;153(1):105–112. doi: 10.1016/j.ahj.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Ypenburg C, Roes SD, Bleeker GB, et al. Effect of total scar burden on contrast-enhanced magnetic resonance imaging on response to cardiac resynchronization therapy. Am J Cardiol. 2007 Mar 1;99(5):657–660. doi: 10.1016/j.amjcard.2006.09.115. [DOI] [PubMed] [Google Scholar]
- 34.Bleeker GB, Kaandorp TA, Lamb HJ, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006 Feb 21;113(7):969–976. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]
- 35.Gorcsan J., 3rd Is the magnet a better crystal ball for predicting response to cardiac resynchronization therapy? JACC Cardiovasc Imaging. 2008 Sep;1(5):569–571. doi: 10.1016/j.jcmg.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Sipahi I, Carrigan TP, Rowland DY, Stambler BS, Fang JC. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Arch Intern Med. 2011 Sep 12;171(16):1454–1462. doi: 10.1001/archinternmed.2011.247. Erratum in: Arch Intern Med. 2011 Sep 12;171(16):1429. [DOI] [PubMed] [Google Scholar]
- 37.Lecoq G, Leclercq C, Leray E, et al. Clinical and electrocardiographic predictors of a positive response to cardiac resynchronization therapy in advanced heart failure. Eur Heart J. 2005 Jun;26(11):1094–1100. doi: 10.1093/eurheartj/ehi146. [DOI] [PubMed] [Google Scholar]
- 38.Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008 May 20;117(20):2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 39.Bax JJ, Gorcsan J., 3rd Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol. 2009 May 26;53(21):1933–1943. doi: 10.1016/j.jacc.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 40.Bilchick KC, Dimaano V, Wu KC, et al. Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2008 Sep;1(5):561–568. doi: 10.1016/j.jcmg.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White JA, Yee R, Yuan X, et al. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006 Nov 21;48(10):1953–1960. doi: 10.1016/j.jacc.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 42.Singh JP, Klein HU, Huang DT, et al. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation. 2011 Mar 22;123(11):1159–1166. doi: 10.1161/CIRCULATIONAHA.110.000646. [DOI] [PubMed] [Google Scholar]
- 43.Spragg DD, Dong J, Fetics BJ, et al. Optimal left ventricular endocardial pacing sites for cardiac resynchronization therapy in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2010 Aug 31;56(10):774–781. doi: 10.1016/j.jacc.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Ginks MR, Lambiase PD, Duckett SG, et al. A simultaneous X-Ray/MRI and noncontact mapping study of the acute hemodynamic effect of left ventricular endocardial and epicardial cardiac resynchronization therapy in humans. Circ Heart Fail. 2011 Mar;4(2):170–179. doi: 10.1161/CIRCHEARTFAILURE.110.958124. [DOI] [PubMed] [Google Scholar]
- 45.Morgan JM, Biffi M, Gellér LA, et al. 35th Annual Scientific Sessions. Heart Rhythm Society. 5. Vol. 11. San Francisco, CA: Heart Rhythm; 2014. May, Feasibility and safety results of a novel superior-access, atrial transseptal approach to left ventricular endocardial lead implantation: one month follow-up of the alternate site cardiac resynchronization (ALSYNC) study; pp. S40–S41. abstract AB17-03. May 7-10, 2014. (suppl) [Google Scholar]
- 46.Wieneke H, Konorza T, Erbel R, Kisker E. Leadless pacing of the heart using induction technology: a feasibility study. Pacing Clin Electrophysiol. 2009 Feb;32(2):177–183. doi: 10.1111/j.1540-8159.2008.02200.x. [DOI] [PubMed] [Google Scholar]
- 47.Benditt DG, Goldstein M, Belalcazar A. The leadless ultrasonic pacemaker: a sound idea? Heart Rhythm. 2009 Jun;6(6):749–751. doi: 10.1016/j.hrthm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Mullens W, Grimm RA, Verga T, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009 Mar 3;53(9):765–773. doi: 10.1016/j.jacc.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 49.Mullens W, Kepa J, De Vusser P, et al. Importance of adjunctive heart failure optimization immediately after implantation to improve long-term outcomes with cardiac resynchronization therapy. Am J Cardiol. 2011 Aug 1;108(3):409–415. doi: 10.1016/j.amjcard.2011.03.060. [DOI] [PubMed] [Google Scholar]
- 50.Martin DO, Lemke B, Birnie D, et al. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm. 2012 Nov;9(11):1807–1814. doi: 10.1016/j.hrthm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Brugada J, Brachmann J, Delnoy PP, et al. Automatic optimization of cardiac resynchronization therapy using SonR-rationale and design of the clinical trial of the SonRtip lead and automatic AV-VV optimization algorithm in the paradym RF SonR CRT-D (RESPOND CRT) trial. Am Heart J. 2014 Apr;167(4):429–436. doi: 10.1016/j.ahj.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Upadhyay GA, Choudhry NK, Auricchio A, Ruskin J, Singh JP. Cardiac resynchronization in patients with atrial fibrillation: a meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2008 Oct 7;52(15):1239–1246. doi: 10.1016/j.jacc.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 53.Gasparini M, Auricchio A, Regoli F, et al. Four-year efficacy of cardiac resynchronization therapy on exercise tolerance and disease progression: the importance of performing atrioventricular junction ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2006 Aug 15;48(4):734–743. doi: 10.1016/j.jacc.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 54.Sweeney MO. Wide right. Heart Rhythm. 2005 Jun;2(6):616–618. doi: 10.1016/j.hrthm.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Haghjoo M, Bagherzadeh A, Farahani MM, Haghighi ZO, Sadr-Ameli MA. Significance of QRS morphology in determining the prevalence of mechanical dyssynchrony in heart failure patients eligible for cardiac resynchronization: particular focus on patients with right bundle branch block with and without coexistent left-sided conduction defects. Europace. 2008 May;10(5):566–571. doi: 10.1093/europace/eun081. [DOI] [PubMed] [Google Scholar]
- 56.Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) Circulation. 2011 Mar 15;123(10):1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 57.Strauss DG, Selvester RH. The QRS complex––a biomarker that “images” the heart: QRS scores to quantify myocardial scar in the presence of normal and abnormal ventricular conduction. J Electrocardiol. 2009 Jan-Feb;42(1):85–96. doi: 10.1016/j.jelectrocard.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 58.Lund LH, Jurga J, Edner M, et al. Prevalence, correlates, and prognostic significance of QRS prolongation in heart failure with reduced and preserved ejection fraction. Eur Heart J. 2013 Feb;34(7):529–539. doi: 10.1093/eurheartj/ehs305. [DOI] [PubMed] [Google Scholar]
- 59.Chung ES, Katra RP, Ghio S, et al. Cardiac resynchronization therapy may benefit patients with left ventricular ejection fraction >35%: a PROSPECT trial substudy. Eur J Heart Fail. 2010 Jun;12(6):581–587. doi: 10.1093/eurjhf/hfq009. [DOI] [PubMed] [Google Scholar]