Abstract
Serotonin produced by neuroendocrine tumors is believed to be a principal cause of the diarrhea in carcinoid syndrome. We assessed the safety and efficacy of telotristat etiprate, an oral serotonin synthesis inhibitor, in patients with diarrhea associated with carcinoid syndrome. In this prospective, randomized study, patients with evidence of carcinoid tumor and ≥4 bowel movements (BMs)/day despite stable-dose octreotide LAR depot therapy were enrolled in sequential, escalating, cohorts of 4 patients/cohort. In each cohort, 1 patient was randomly assigned to placebo and 3 patients to telotristat etiprate, at 150, 250, 350, or 500 mg 3x/day (tid). In a subsequent cohort, 1 patient was assigned to placebo and 6 patients to telotristat etiprate 500 mg tid. Patients were assessed for safety, BM frequency (daily diary), 24-hour urinary 5-hydroxyindoleacetic acid (u5-HIAA), and adequate relief of carcinoid gastrointestinal symptoms (using a weekly questionnaire). Twenty-three patients were treated; 18 received telotristat etiprate and 5 received placebo. Adverse events were generally mild. Among evaluable telotristat etiprate-treated patients, 5/18 (28%) experienced a ≥30% reduction in BM frequency for ≥2 weeks, 9/16 (56%) experienced biochemical response (≥50% reduction or normalization in 24-hour u5-HIAA) at Week 2 or 4, and 10/18 (56%) reported adequate relief during at least 1 of the first 4 weeks of treatment. Similar activity was not observed in placebo-treated patients. Telotristat etiprate was well tolerated. Our observations suggest that telotristat etiprate has activity in controlling diarrhea associated with carcinoid syndrome. Further studies confirming these findings are warranted.
Keywords: adult, carcinoid syndrome, diarrhea, neuroendocrine tumor, serotonin, tryptophan hydroxylase
INTRODUCTION
Carcinoid syndrome is characterized by watery diarrhea, episodic flushing, bronchoconstriction, and eventually, the development of right-sided valvular heart disease. The symptoms of carcinoid syndrome have been attributed, in part, to elevated levels of 5-HT (serotonin) secreted by the tumor.(Kvols et al. 2012; Druce et al. 2009) Serotonin appears to play a particularly important role in the development of carcinoid-related diarrhea. In an early study, treatment with the serotonin receptor antagonist methysergide was reported to reduce the frequency of diarrhea in patients with carcinoid syndrome.(Melmon et al. 1965) In a second study published in 1967, inhibition of serotonin synthesis with the drug parachlorophenylalanine (pCPA) resulted in substantial improvement of diarrhea in patients with carcinoid syndrome. The further use of either drug, however, was precluded by the development of psychiatric side effects.(Engleman et al. 1967) The development of carcinoid heart disease is likely mediated by serotonin.(Creutzfeldt 1996); Møller et al. 2003; Dobson et al. 2013; Gustafsson et al. 2005) Evidence supporting the role of serotonin was previously demonstrated (CDC 1997; Connolly 1997); individuals treated with the serotonin agonist fenfluramine developed cardiac lesions identical to those observed in patients with longstanding carcinoid syndrome.
In current practice, patients with carcinoid syndrome are generally treated with somatostatin analogs (SSAs), given by injection. The effects of SSAs are mediated by somatostatin receptors (predominately receptor subtype 2), which have an inhibitory effect on tumor secretion of serotonin and other neuropeptides into the systemic circulation. In an initial study, the subcutaneous administration of the SSA octreotide, administered at a dosage of 150 µg 3 times a day (tid), improved the symptoms of carcinoid syndrome in 88% of patients.(Kvols et al. 1986) A long-acting depot form of octreotide, which can be administered on a monthly basis, is now commonly used in patients with carcinoid syndrome, together with use of short-acting octreotide as needed for breakthrough symptoms. Lanreotide, another SSA, appears to be similar to octreotide in its clinical efficacy. Over time, however, patients may develop tachyphylaxis to the effects of SSAs, or may respond to a lesser extent due to increased tumor burden.(Kvols et al. 2012) There are few treatment options presently available for these patients, and new therapies are needed.
Telotristat etiprate is an oral, systemically available, small-molecule inhibitor of peripheral serotonin synthesis. Telotristat etiprate acts by inhibiting tryptophan hydroxylase, the rate limiting enzyme in the conversion of tryptophan to serotonin. In multiple-dose PK studies, the median Tmax after telotristat etiprate (at dose levels ranging from 100 mg to 500 mg) ranged from 2 to 4 hours on both Day 1 and Day 14. The T1/2 of LP-778902 after multiple doses of telotristat etiprate ranged from 3.65 to 11.7 hours, consistent with dosing tid.
The molecule was designed not to cross the blood-brain barrier at the intended dose, and preclinical studies suggested that telotristat etiprate acts primarily peripherally, with little, if any, activity observed in the central nervous system.(Lexicon 2007), unpublished observations) Phase I studies in healthy volunteers demonstrated that telotristat etiprate, administered orally at doses up to 500 mg tid, reduced serotonin production, as measured by urinary 5-hydroxyindoleacetic acid (u5-HIAA), a serotonin metabolite.(Lexicon 2012), unpublished observations) No obvious neurologic or psychiatric side effects were observed in these studies, and other adverse events were infrequent.
In light of the association between excess serotonin production and carcinoid syndrome, we explored the safety and efficacy of telotristat etiprate in patients with carcinoid syndrome and diarrhea inadequately controlled by octreotide in a prospective, randomized study.
PATIENTS AND METHODS
Study Design
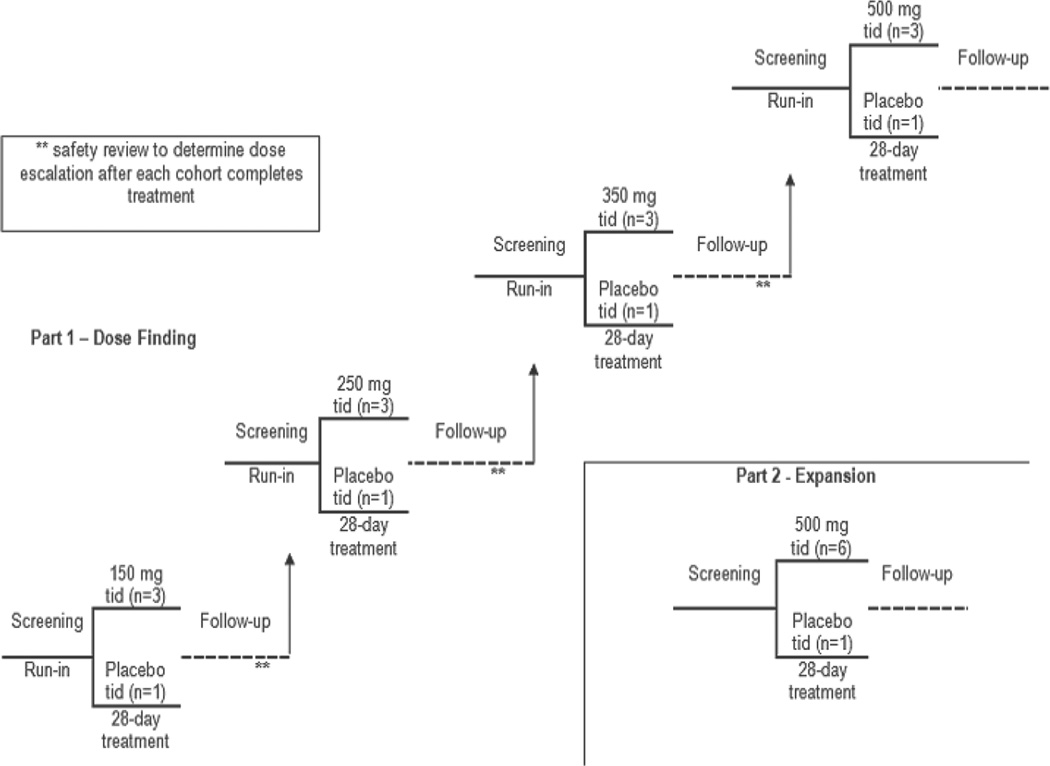
The primary objective of this prospective, randomized study was to assess the safety of telotristat etiprate in patients with carcinoid syndrome and diarrhea inadequately controlled by octreotide. Because of the small sample size, efficacy analyses were considered exploratory. Per protocol, in the dose-escalation phase of the trial, 4 patients were randomized (1 to placebo and 3 to active drug) in each ascending dose cohort of 150, 250, 350, and 500 mg tid, and evaluated for toxicity (Figure 1). Dose escalation to the next cohort was permitted only if ≤2 patients in the cohort experienced a dose-limiting toxicity and after additional review of all additional toxicities experienced by patients in the previous cohort. Then, in the expansion phase of the trial, an additional cohort of 7 patients (6 telotristat etiprate, 1 placebo) was studied at either the maximum tolerated dose (MTD) or at the maximum defined dose in the study (500 mg tid). The goal of the expansion phase was to gain additional clinical experience at the maximum defined dose of telotristat etiprate. The dose-escalation phase and the expansion phase were conducted under the same conditions, with the same assessments of safety and efficacy. Randomization of all patients was central, via an Interactive Voice Response System (IVRS).
Figure 1.
Five cohorts of patients with carcinoid syndrome were sequentially enrolled in the study. The first 4 cohorts ranged in telotristat etiprate dose from 150 mg tid to 500 mg tid, with 4 patients in each cohort (3 patients randomized to active drug, 1 on placebo). The 5th cohort enrolled an additional 7 patients (6 patients randomized to the 500 mg tid dose, 1 to placebo).
Following completion of the initial 4-week blinded treatment period, all eligible patients were offered enrollment into an open-label extension phase (entry at assigned dose level with opportunity to progress to higher dose levels). The results of the blinded treatment period are described here.
This study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the respective Institutional Review Board with jurisdiction over each site. All patients gave written informed consent after receiving a full explanation of the purpose and nature of the study as well as all study-related procedures, and before being enrolled into the study.
Patient Selection
Eligible patients were required to have biopsy-proven metastatic neuroendocrine (carcinoid) tumor and diarrhea inadequately controlled by octreotide therapy. Inadequately controlled diarrhea was defined as an average of ≥4 bowel movements (BM)/day while on stable-dose octreotide LAR depot therapy for at least 3 months; in addition, patients were allowed to use supplemental short-acting octreotide therapy during the run-in and treatment period. Serum creatinine <1.5x the upper limit of normal (ULN), hepatic transaminases <2×ULN, alkaline phosphatase <1.5xULN and total bilirubin within normal limits were required to enter the study.
Patients were excluded if they had a history of short bowel syndrome, more than 12 high-volume, watery BMs/day, or a Karnofsky Performance Status ≤70%. Patients were excluded if they had concomitant use of antidiarrheal agents, anticholinergic antidepressants, opioid analgesic drugs, or drugs specifically affecting bowel motility during the run-in period and for the duration of the study unless, in the opinion of the investigator, discontinuation of such medications would place the subject at unnecessary health risk, and the use of these concomitant medications had been stable for at least 3 months prior to the run-in period. Specific data on frequency of antidiarrheal use after study entry was not obtained.
Assessment of Adverse Events and Dose Limiting Toxicities
In advance of beginning the study, toxicities were predefined as mild (not sufficient to interfere with usual activity), moderate (severe enough to interfere with usual activity), or severe (preventing subjects from performing their usual activities). Adverse events were recorded based on standard reporting criteria. Dose limiting toxicity was defined as ALT ≥4 X ULN (if normal at baseline) or ≥2 X baseline (if above normal at baseline), total bilirubin ≥2 X ULN, alkaline phosphatase ≥3 X ULN (if normal at baseline) or ≥2 X ULN (if above normal at baseline), or constipation, defined as more than 3 consecutive days without a BM.
Assessment of Efficacy
Efficacy was explored using a responder analysis across several endpoints, including BM frequency, change in 24-hour u5-HIAA, and patient-reported adequate relief of symptoms. Patients initially completed a run-in period in which baseline values including BM frequency, flushing frequency, and use of short-acting subcutaneous octreotide as needed for acute symptoms (also known as “rescue” octreotide) were recorded (Table 1).
Table 1.
Baseline Characteristics
| Number of eligible patients | 23 | |
|---|---|---|
| Age | ||
| Mean, years | 62 | |
| Minimum, maximum | 44, 83 | |
| Number and percentage (%) of patients |
||
| Sex | ||
| Male | 11 | (47.8) |
| Female | 12 | (52.2) |
| BM Frequency | ||
| Mean, #/day | 6.3 | |
| Minimum, maximum | 4, 10 | |
| Octreotide dose: | ||
| 30 mg q 4 wks | 4 | (17.4) |
| 30 mg q 3 wks | 2 | (8.7) |
| 40 mg q 2–4 wks | 15 | (65.2) |
| 60 mg q 3 wks | 1 | (4.3) |
| Octreotide infusion pump | 1 | (4.3) |
| Urinary 5-HIAA, mg/24h (range) | ||
| Mean, mg/24-hr | 65 | |
| Minimum, maximum | 0.3, 246 | |
| Primary Tumor Site | ||
| Midgut | 22 | (95.7) |
| Hindgut | 1 | (4.3) |
BM frequency was assessed using a daily patient report via IVRS system. A clinical response in BM frequency was defined either as a ≥30% reduction from baseline in the daily mean number of BMs/week for ≥2 weeks or as achievement of ≤3 BMs/day averaged across the daily values for the week, in the absence of rescue octreotide use.
Baseline 24-hour u5-HIAA was obtained on the day prior to treatment; subsequently, 24-hour urine collections for u5-HIAA were obtained at Weeks 2 and 4 of treatment. Biochemical response was defined either as a ≥50% decrease in 24-hour u5-HIAA levels from baseline, or as normalization of u5-HIAA (in patients who had elevated baseline levels) at either Week 2 or 4. In addition, there must have been absence of octreotide rescue treatment in the week preceding the postdose value when response was achieved.
Patient-reported adequate relief was assessed weekly via IVRS, using responses to the question, “In the past 7 days, have you had adequate relief of your carcinoid syndrome bowel complaints such as diarrhea, urgent need to have a bowel movement, abdominal pain, or discomfort?” Other efficacy endpoints included changes in stool consistency, urgency to defecate, abdominal pain, frequency of flushing, and use of short-acting rescue octreotide.
STATISTICAL CONSIDERATIONS
Descriptive methods were used to summarize the study data. These statistics included sample size (N), mean, standard deviation, median, minimum, and maximum values for continuous variables and counts with related percentages for categorical variables. Statistical tests were conducted in an exploratory manner to help guide inferences for a limited number of efficacy variables. Maximum likelihood methods were used to derive all point estimates of treatment effect. For continuous variables this was the difference between treatment group least squares means derived from analysis of covariance statistics and the difference between treatment group proportions for binomial variables. All analyses were based on the observed data; imputation for missing data points was not performed.
Data was summarized by treatment group. The placebo group consisted of patients pooled from the dose-escalation phase and the expansion phase of the study. In a like manner, patients treated with the telotristat etiprate dose selected from the dose-escalation phase were pooled with patients receiving that same dose in the expansion phase; making a single dose group at 500 mg tid. All other telotristat etiprate treatment groups were summarized as planned from the dose-escalation phase of the study. Subjects were included in the treatment group as assigned by the randomization plan.
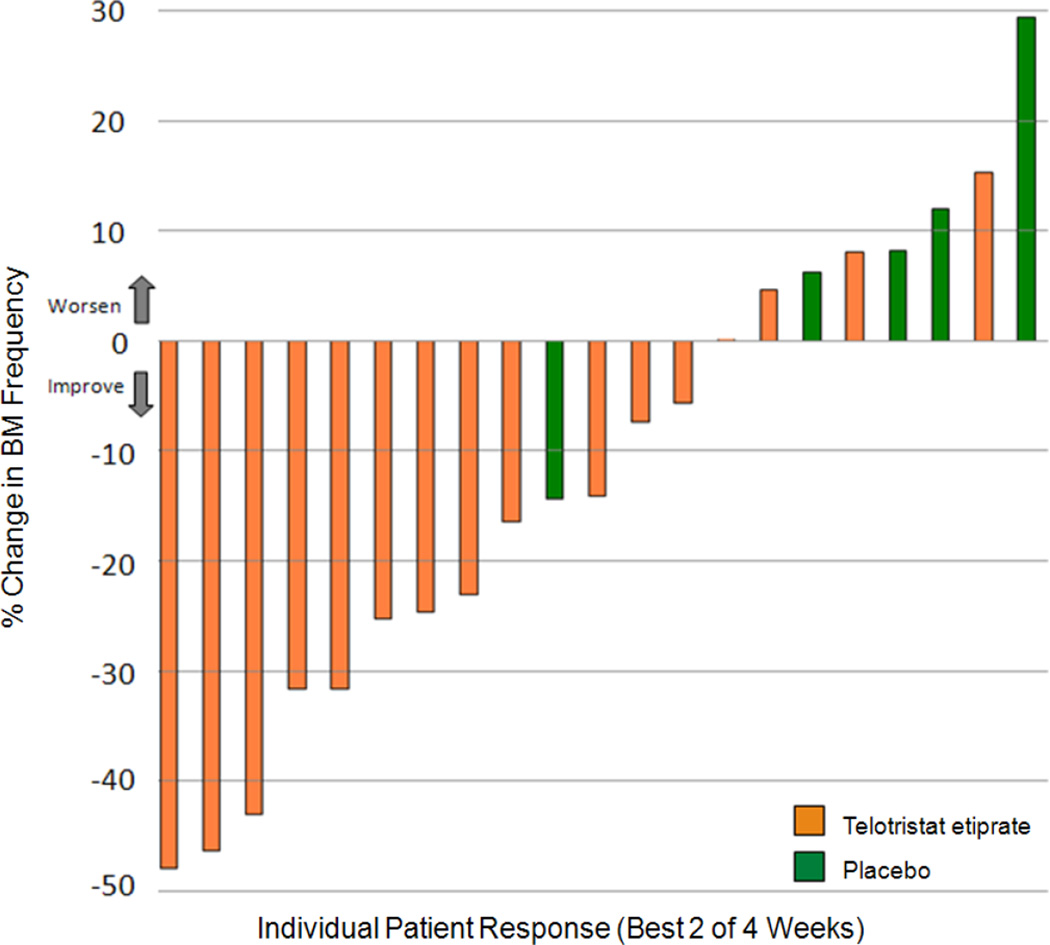
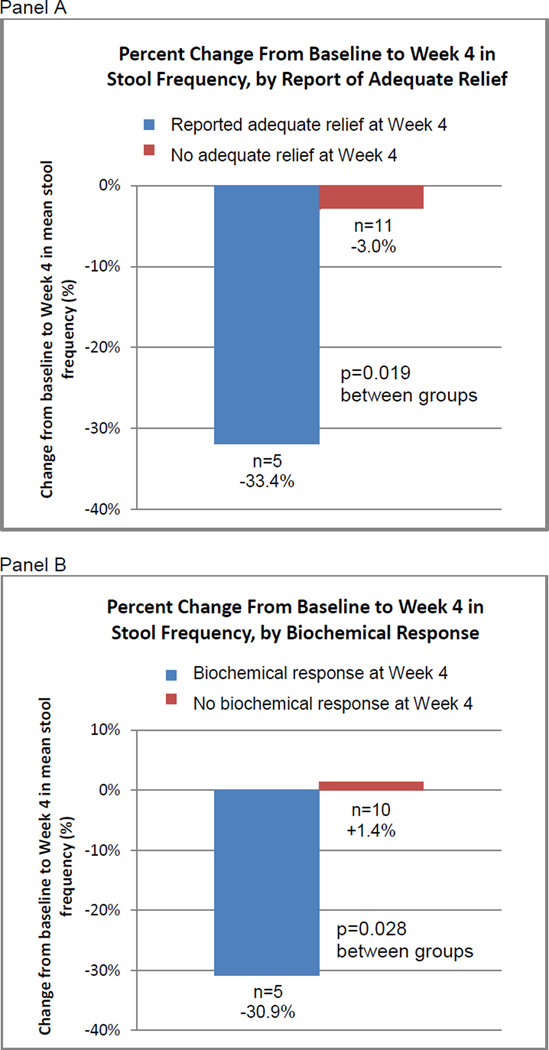
A waterfall plot (Figure 2) was used to visually display the percentage change from baseline in the number of BMs measured over the course of the study for both telotristat etiprate and placebo groups. Subset analyses Wilcoxon 2-sample tests, were performed to explore percentage changes from baseline in BM frequency at Week 4 (Figure 3). Subsets included those patients who reported adequate relief and those who did not as well as those who achieved biochemical response and those who did not.
Figure 2.
The majority of evaluable treated patients showed improvement over baseline that was present 2 or more weeks. Only 1 placebo patient showed improvement over baseline, while the remaining 4 placebo patients showed increasing BM frequency.
Figure 3.
Reduction in BMs/day was greater among patients who reported adequate relief at Week 4, and was also greater in patients who had biochemical response at Week 4, defined as a 50% or greater reduction from baseline in u5-HIAA.
All analyses were performed, and summaries were prepared, using SAS (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
A total of 23 patients were randomly assigned, (18 to telotristat etiprate and 5 to placebo), and treated at 11 clinical sites. Study design and patient disposition are summarized in Figure 1; baseline demographics are described in Table 1. All but 1 patient had midgut NET. At baseline, 65% of patients were on standard antidiarrheal medications (eg, lomotil, loperimide, tincture of opium, and cholestyramine); 87% of those patients on antidiarrheals at baseline either continued or resumed concomitant antidiarrheals during the reported study period. Four patients were receiving octreotide at the maximum approved dose (30 mg IM q 4 weeks); all other patients were receiving octreotide at higher doses.
Safety
Telotristat etiprate was well tolerated at all dose levels. Of the 23 patients who received either telotristat etiprate or placebo, 4/5 (80%) of the placebo patients and 18/18 (100%) of the patients receiving telotristat etiprate reported at least 1 treatment-emergent adverse event (TEAE) (Table 2). There were no serious adverse events (SAEs) in the placebo patients and 2/18 patients receiving telotristat etiprate experienced serious adverse events (SAEs). One of these patients was hospitalized for nausea and vomiting; this patient had a pre-existing history of similar symptoms and subsequently elected to withdraw from the study. The second patient had a recurrence of squamous cell carcinoma on the hand, deemed unlikely treatment-related. In interpreting the incidence of adverse events, it is useful to recall that patients on telotristat etiprate (18) outnumbered placebo patients (5) by a ratio of greater than 3.
Table 2.
Selected Treatment Emergent Adverse Events by Treatment Group
| Placebo n=5 |
150 mg tid n=3 |
250 mg tid n=3 |
350 mg tid n=3 |
500 mg tid n=9 |
|
|---|---|---|---|---|---|
| Number and percentage (%) of patients | |||||
| Patients with any TEAE | 4 (80.0) | 3 (100.0) | 3 (100.0) | 3 (100.0) | 9 (100.0) |
| Mild | 1 (20.0) | 1 (33.3) | 1 (33.3) | 0 (0.0) | 3 (33.3) |
| Moderate | 1 (20.0) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 5 (55.6) |
| Severe | 2 (40.0) | 1 (33.3) | 0 (0.0) | 2 (66.7) | 1 (11.1) |
| Patients with SAEs | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 0 (0.0) |
| Gastrointestinal | |||||
| Diarrhea | 2 (40.0) | 1 (33.3) | 2 (66.7) | 0 (0.0) | 4 (44.4) |
| Nausea | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 4 (44.4) |
| Vomiting | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Abdominal pain | 0 (0.0) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 0 (0.0) |
| Abdominal discomfort | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Constipation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| Abdominal. distension | 1 (20.0) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 1 (11.1) |
| Neurologic | |||||
| Dysgeusia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (22.2) |
| Dizziness | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Headache | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Psychiatric | |||||
| Anxiety | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Depression | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Sleep disorder | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
|
Musculoskeletal and connective tissue |
|||||
| Musculoskeletal pain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| Myalgia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| Metabolism and nutrition | |||||
| Decreased appetite | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (22.2) |
| Laboratory investigations | |||||
| Elevated alkaline phosphatase | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | |||||
| Fatigue | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 2 (22.2) |
| Asthenia | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) |
TEAE: treatment emergent adverse events; SAE: serious adverse events
Most reported adverse events were mild and were gastrointestinal in nature. These included mild nausea, vomiting, or abdominal discomfort. The majority of AEs were transient and resolved during the course of the treatment period or open label extension. Reported psychiatric events were rare; a single patient reported depression while receiving telotristat etiprate. This patient had a preexisting diagnosis of anxiety disorder and the AE resolved while the patient continued to receive study therapy. Elevations of transaminases over baseline during the 4 weeks of the study occurred in both treatment groups and included 3/18 (17%) on telotristat etiprate and 2/5 (40%) on placebo. All elevations were <2 X the patient’s baseline; the majority of elevations were transient. No patient experienced elevations in total bilirubin above the reported normal range during the treatment period.
No clear correlation between dose level and the frequency of adverse events was observed.
Clinical Response/Decrease in BM Frequency
Five (28%) of 18 patients treated with telotristat etiprate and 0 of 5 patients treated with placebo achieved a clinical response, defined either as a ≥30% reduction from baseline in the daily mean number of BMs/week for 2 or more of the 4 weeks on trial.. Of note, these clinical responses included only reductions of BM frequency in the absence of elective, rescue octreotide use.
Most (12/16) telotristat etiprate patients had at least some reduction in BM frequency for 2 or more weeks of the 4-week treatment period, as compared to 1 of 5 placebo patients (Figure 2). At Week 4, mean decreases in BM frequency were observed in all dose cohorts for patients receiving telotristat etiprate; no mean decrease in BM frequency was observed in patients receiving placebo (Table 3).
Table 3.
Extent of Exposure and Efficacy by Treatment Group
| Placebo n=5 |
150 mg tid n=3 |
250 mg tid n=3 |
350 mg tid n=3 |
500 mg* tid n=9 |
Pooled telotristat etiprate n=18 |
|
|---|---|---|---|---|---|---|
| BM Frequency (Clinical Response) | ||||||
| Baseline Mean | 5.3 | 8.0 | 6.9 | 5.8 | 6.5 | 6.3 |
| BM Frequency, #/day (Range) |
(4–8) | (5–10) | (5–9) | (5–7) | (4–9) | (4–10) |
| Clinical Responders n/evaluable (%) |
0/5 (0) | 1/3 (33) | 2/3 (67) | 0/3 (0) | 2/9 (22) | 5/18 (28) |
| Mean change daily BM Frequency Week 4 |
+0.8 | −1.4 | −2.2 | −1.2 | −0.7 | −1.2 |
| Urinary 5-HIAA (Biochemical Response) | ||||||
| Baseline Mean | 100.04 | 51.53 | 2.4 | 3.33 | 105.2 | 56.8 |
| u5-HIAA, mg/24h (Range) |
(0.3–246.0) | (4.6–117.0) | (1.7–3.6) | (2.8–3.9) | (8.4–217.0) | (2.8–217) |
| Biochemical Responders at Week 2 or Week 4 (n/evaluable) |
0/5 (0) | 2/3 (67) | 1/3 (33) | 0/2 (0) | 6/8 (75) | 9/16 (56) |
| Patient-reported adequate relief | ||||||
| Week 1 n/evaluable, (%) |
0/4 (0) | 1/3 (33) | 1/3 (33) | 0/3 (0) | 4/9 (44) | 6/18 (33) |
| Week 2 n/evaluable, (%) |
0/5 (0) | 2/3 (67) | 1/3 (33) | 0/2 (0) | 2/8 (25) | 5/16 (31) |
| Week 3 n/evaluable, (%) |
0/5 (0) | 1/2 (50) | 1/3 (33) | 1/2 (50) | 2/8 (25) | 5/15 (33) |
| Week 4 n/evaluable, (%) |
0/4 (0) | 1/2 (50) | 2/3 (67) | 1/2 (50) | 2/6 (33) | 6/13 (46) |
One patient from the 500 mg group was excluded from u5-HIAA baseline and Week 4 value calculation and included in the biochemical response calculation based on prespecified definitions of timing of u5-HIAA collection.
Based on the analysis of a related measure of activity of complete response, (ie, less than 4 bowel movements per day averaged over the week, a ≥50% from decrease from baseline in daily bowel movements averaged over the week, or a positive global assessment for the last 2 weeks of treatment), telotristat etiprate-treated patients again showed a favorable outcome compared to those on placebo; 6 of 8 patients treated with telotristat etiprate achieved a complete response at Week 4 compared to a rate of 0% in placebo-treated patients (0 of 5) at Week 4.
Biochemical Response
Nine of 16 evaluable telotristat etiprate patients and 0 of 5 placebo patients achieved a biochemical response in u5-HIAA (normal, ≤6 mg/24 hours) , defined either as a ≥50% decrease in 24-hour u5-HIAA levels from baseline, or as normalization of u5-HIAA (in patients who had elevated baseline levels) at either Week 2 or 4 (Table 3).
Adequate Relief
As described above, patient-reported adequate relief was collected weekly using IVRS during the 4-week active treatment period. Data was available for 80/92 weekly patient assessments (Table 3). During this period, no patients receiving placebo reported adequate relief, whereas 10/18 (56%) of the telotristat etiprate-treated patients reported relief at 1 or more weekly time points. At the end of the blinded treatment period (Week 4), 4 patients receiving placebo and 13 patients receiving telotristat etiprate completed the assessment of adequate relief. None of these 4 patients receiving placebo reported adequate relief whereas 6/13 (46%) receiving telotristat etiprate reported relief.
Associations Between Efficacy Parameters
We retrospectively explored associations between decreases in BM frequency and patient-reported adequate relief (Figure 3, Panel A). We focused our analysis on Week 4 (end of treatment period) data, for which 15 patients were evaluable. Patients who reported adequate relief at Week 4, regardless of treatment, experienced a 33.4% mean reduction in BM frequency during Week 4 (n=5), from pretreatment baseline. Patients who did not report adequate relief at Week 4 had a 3.0% mean decrease in mean BM frequency (n=10). The difference between these 2 groups, based on achieving adequate relief, was significant (p=0.019).
Similarly, we explored associations between decreases in BM frequency and biochemical response, defined here as a 50% or greater reduction in u5-HIAA at Week 4 (Figure 3, Panel B). Fifteen patients were evaluable in this analysis. Patients with biochemical response at Week 4 experienced a mean 30.9% reduction in mean BM frequency during Week 4 (n=5), compared with baseline. In contrast, patients who did not have biochemical response at Week 4 had a 1.4% mean increase in mean BM frequency (n=10). The difference between these 2 groups, based on achieving a biochemical response, was significant (p=0.028).
Clinical responses in BM frequency and in adequate relief were found among patients with elevated u5-HIAA at baseline and also among those with normal baseline u5-HIAA. Of the 11 patients who entered the study with elevated baseline u5-HIAA levels, 3 (27%) were clinical responders, and 2 (18%) reported adequate relief at week 4. Of the 10 patients who entered the study with u5-HIAA values in the normal range, 2 (20%) were clinical responders, and 3 (30%) reported adequate relief at Week 4.
Other Endpoints
No clear differences in use of rescue octreotide between patients treated with telotristat and placebo were observed. No clear differences in stool consistency, urgency to defecate, abdominal pain, or frequency of flushing were observed between patients treated with telotristat etiprate or those receiving placebo.
DISCUSSION
Excess serotonin production has been implicated as a causative factor in carcinoid-associated diarrhea and other manifestations of the carcinoid syndrome.(Druce et al. 2009) In carcinoid syndrome, increased frequency of BMs has been associated with a decreased quality of life.(Beaumont et al. 2012) Our results provide evidence supporting the safety of telotristat etiprate, a tryptophan hydroxylase inhibitor. Our observations further suggest that telotristat etiprate may have promising clinical activity in patients with carcinoid syndrome and diarrhea inadequately controlled by octreotide.
Overall, at all doses, including the maximum dose tested (500 mg tid), telotristat etiprate was safe and well tolerated, as evidenced by the comparable occurrence of AEs between placebo and telotristat dose groups. In our 4-week study, CNS side effects were rare. Decades ago, an association between excess serotonin production and carcinoid syndrome was supported by observations that inhibition of serotonin with pCPA improved symptoms in patients with carcinoid syndrome.(Engelman et al. 1967) However, pCPA crossed the blood-brain barrier, resulting in severe psychological impairment. In contrast, telotristat etiprate was designed not to cross the blood-brain barrier at the intended therapeutic dose, and acts only peripherally. In addition, preclinical models suggested that telotristat etiprate may slow peristalsis (data not shown),(Lexicon 2007), unpublished observations), which could contribute to intermittent abdominal discomfort, nausea, and bloating. However, although we observed occasional instances of these symptoms in this study, the events were generally mild and resolved with continued treatment.
While our study was not powered to assess efficacy formally, several prespecified analyses in the study suggest that telotristat etiprate has clinical activity in patients with carcinoid syndrome and diarrhea inadequately controlled by octreotide. Among evaluable telotristat etiprate treated patients, 28% experienced a decrease in BM frequency of 30% or more, 56% experienced ≥50% decrease or normalization of 24-hour u5-HIAA levels, and 56% reported adequate relief of symptoms at least once during the assessment period. Similar evidence of efficacy was not observed in any of the placebo treated patients. Furthermore, in retrospective analyses, mean reductions in BM frequency were numerically greater in those with biochemical responses in u5-HIAA and in patient reports of symptom relief, endpoints achieved only with telotristat etiprate and not with placebo.
An underlying assumption in our decision to evaluate telotristat etiprate in carcinoid syndrome patients was that the symptoms of diarrhea were mediated by serotonin,(Druce et al. 2009) and that a decrease in serotonin production would therefore result in improvement in these symptoms. The clinical responses seen in patients treated by telotristat etiprate suggest that this assumption is correct.
Not all symptomatic patients with diagnoses of carcinoid syndrome entering the study had elevated u5-HIAA levels at baseline, and patients with normal u5-HIAA at baseline showed improvements in clinical parameters similar to those seen in patients with elevated u5-HIAA. Urinary 5-HIAA levels can be affected by variations in diet and inconsistencies in 24-hour urine collections. Alternatively, while the protocol required a diagnosis of carcinoid syndrome and excluded patients with short bowel syndrome, it is possible that some of the patients with normal u5-HIAA at baseline had diarrhea on the basis of other serotonin-mediated conditions. Finally, somatostatin analogs decrease serotonin secretion, which results in decreased systemic 5-HIAA levels. Urinary 5-HIAA levels may, therefore, be an imperfect predictor of serotonin-mediated diarrhea in patients who are already receiving treatment with somatostatin analogs.
In conclusion, in this preliminary study, telotristat etiprate exhibited a favorable safety profile and was well tolerated in these patients with carcinoid syndrome. Evidence of clinical benefit was observed in this study across several endpoints, including decreased BM frequency, decreased u5-HIAA levels, and patient-reported adequate relief of symptoms. Further studies of telotristat etiprate in patients with carcinoid-related diarrhea are warranted.
Acknowledgements
The authors wish to thank Kristi A. Boehm, MS, ELS for her writing and editing assistance; Shanna Jackson, MBA for her study monitoring; Johanna Bronner, BS for data management, and Melissa Yang, PhD for data review.
Funding
This study was funded in whole by Lexicon Pharmaceuticals, Inc.
Footnotes
ClinicalTrials.gov registry # NCT00853047
Results have been presented in part at the North American Neuroendocrine Tumor Society, 2011 annual meeting (October 20–22, Minneapolis, MN), the American Society of Clinical Oncology, 2012 GI symposium (January 19–22, San Francisco, CA), and the 2012 European Neuroendocrine Tumor Society Annual Meeting, Copenhagen.
DECLARATION OF INTEREST
Declaration of Interest
MHK, TO, AP, EB, JCY, and LK have received payment for their time spent conducting study-related tasks. MHK has received paid honoraria from Lexicon Pharmaceuticals, Inc.
PB, PL, BZ, DF, and AS are currently employees of Lexicon Pharmaceuticals, Inc., and own stock.
LL, JF, KF, and JJ were employees of Lexicon Pharmaceuticals, Inc., at the time the study was conducted and may currently own stock.
AS is on the Board of Directors for Lexicon Pharmaceuticals, Inc.
Author contributions
Study design: MHK, PB, JF, KF, JJ, BZ, AS
Provided data: MHK, TO, AP, EB, JCY, LY
Review of data: MHK, TO, AP, EB, LL, PB, JF, KF, JJ, JCY, LK, PL, BZ, DF, AS
Statistical analysis: PB
Manuscript writing: MHK, TO, AP, EB, LL, PB, JF, KF, JJ, JCY, LK, PL, BZ, DF, AS
Approval of final manuscript: MHK, TO, AP, EB, LL, PB, JF, KF, JJ, JCY, LK, PL, BZ, DF, AS
REFERENCES
- Beaumont JL, Cella D, Phan AT, Choi S, Liu Z, Yao JC. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas. 2012 Apr;41(3):461–466. doi: 10.1097/MPA.0b013e3182328045. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: U.S. Department of Health and Human Services interim public health recommendations, November 1997. MMWR Morb Mortal Wkly Rep. 1997 Nov 14;46(45):1061–1066. [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997 Aug 28;337(9):581–588. doi: 10.1056/NEJM199708283370901. Erratum in: N Engl J Med 1997 Dec 11;337(24): 1783. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W. Carcinoid tumors: development of our knowledge. World J Surg. 1996;20:126–131. doi: 10.1007/s002689900020. [DOI] [PubMed] [Google Scholar]
- Dobson R, Burgess MI, Banks M, et al. The association of a panel of biomarkers with the presence and severity of carcinoid heart disease: a cross-sectional study. PLoS One. 2013:e73679. doi: 10.1371/journal.pone.0073679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druce M, Rockall A, Grossman AB. Fibrosis and carcinoid syndrome: from causation to future therapy. Nat Rev Endocrinol. 2009 May;5(5):276–283. doi: 10.1038/nrendo.2009.51. [DOI] [PubMed] [Google Scholar]
- Engelman K, Lovenberg W, Sjoerdsma A. Inhibition of serotonin synthesis by para-chlorophenylalanine in patients with the carcinoid syndrome. N Engl J Med. 1967 Nov 23;277(21):1103–1108. doi: 10.1056/NEJM196711232772101. [DOI] [PubMed] [Google Scholar]
- Gustafsson BI, Tømmerås K, Nordrum I, et al. Long-term serotonin administration induces heart valve disease in rats. Circulation. 2005 Mar 29;111(12):1517–1522. doi: 10.1161/01.CIR.0000159356.42064.48. [DOI] [PubMed] [Google Scholar]
- Kvols LK, Moertel CG, O'Connell MJ, Schutt AJ, Rubin J, Hahn RG. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986 Sep 11;315(11):663–666. doi: 10.1056/NEJM198609113151102. [DOI] [PubMed] [Google Scholar]
- Kvols L, Oberg KE, O'Dorisio T, et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a Phase II study. Endocr Relat Cancer. 2012;19(5):657–666. doi: 10.1530/ERC-11-0367. [DOI] [PubMed] [Google Scholar]
- Lexicon in-house Report Number P103-002. Pharmacology of LX1606. 2007. Nov 30, [Google Scholar]
- Lexicon in-house LX1606.1-202 clinical study report. 2012. May 01, [Google Scholar]
- Melmon KL, Sjoerdsma A, Oates JA, Laster L. Treatment of malabsorption and diarrhea of the carcinoid syndrome with methysergide. Gastroenterology. 1965 Jan;48:18–24. [PubMed] [Google Scholar]
- Møller JE, Connolly HM, Rubin J, Seward JB, Modesto K, Pellikka PA. Factors associated with progression of carcinoid heart disease. N Engl J Med. 2003;348:1005–1015. doi: 10.1056/NEJMoa021451. [DOI] [PubMed] [Google Scholar]