Figure 3. Cellular events controlling intracellular α-synuclein levels and possible therapeutic strategies to combat α-synuclein accumulation and transmission.
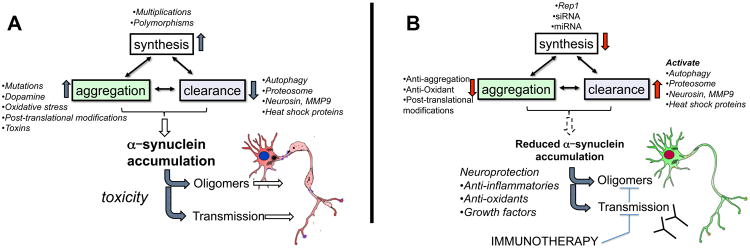
Intracellular α-synuclein (α-syn) levels are tightly regulated by the balance between the rates of α-syn synthesis, clearance and aggregation. a | Abnormalities affecting α-syn synthesis, including SNCA multiplication and polymorphisms, may increase intracellular α-syn levels and induce its accumulation. Accumulation may also be caused by a failure to degrade α-syn. Clearance deficits might arise from failure of the ubiquitin – proteasome system, chaperone-mediated autophagy dysfunction (induced by Parkinson disease-linked mutations) or dysfunction of proteases (neurosin or matrix metalloprotease 9). Finally, certain SNCA mutations, post-translational modifications, oxidative stress, toxins and interaction with oxidized dopamine increase the propensity of α-syn to aggregate and accumulate. b | Targeted mechanisms to reduce α-syn accumulation include decreasing protein synthesis by using Rep1, siRNA or miRNA. Accumulation may also be decreased by activating mechanisms or proteins involved in clearance, such as autophagy, the proteasome, neurosin, MMP9 and heat shock proteins. Additionally, aggregation of α-syn can be decreased using anti-aggregating, antioxidant, or post-translational modification approaches. Finally, immunotherapy may be used to block transmission and oligomer formation.
