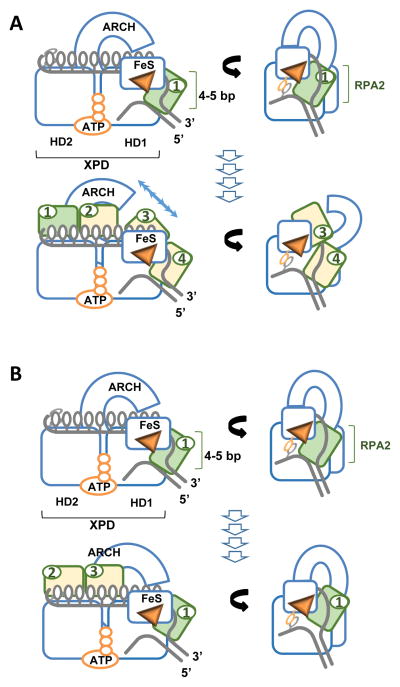
Figure 6. Building an active helicase.
Two models for cooperation between XPD and RPA2 A. Scenario 1: RPA2 (depicted in green) binds at the ss-dsDNA junction, destabilizes 4–5 bp dsDNA upstream of the helicase. XPD then advances into the melted region trapping the released strands. Bound RPA2 moves with the translocating strand through the motor core of XPD. The cycle repeats with the next RPA2 molecule (yellow 2&3) binding at the junction. The prerequisite for this scenario is opening of the ARCH domain sufficient to accommodate RPA2-ssDNA complex. B. Alternatively, The same molecule of RPA2 may remain associated with the helicase jammed between the junction and the hole through which the translocating strand passes. Additional RPA2 molecules (2&3) may trap the unwound strands behind the helicase.