Abstract
Femoral head separation (FHS) is an idiopathic bone problem that causes lameness and production losses in commercial poultry. In a model of prednisolone-induced susceptibility to FHS, the changes in plasma proteins and peptides were analyzed to find possible biomarkers. Plasma samples from control and FHS-susceptible birds were depleted of their high abundance proteins by acetonitrile precipitation and were then subjected to cation exchange and reverse-phase (RP) fractionations. Analysis with matrix assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF-MS) showed several differentially expressed peptides, two of which were isolated by RP-HPLC and identified as the fragments of apolipoprotein A-I. The acetonitrile fractionated plasma proteins were subjected to reduction/alkylation and trypsin digestion followed by liquid chromatography and tandem mass spectrometry, which showed the absence of protocadherin 15, vascular endothelial growth factor-C, and certain transcription and ubiquitin-mediated proteolytic factors in FHS-prone birds. It appears that prednisolone-induced dyslipidemia, vascular, and tissue adhesion problems may be consequential to FHS. Validity of these biomarkers in our model and the natural disease must be verified in future using traditional approaches.
BIOMARKER INSIGHTS
Lameness because of femoral head separation (FHS) is a production and welfare problem in the poultry industry. Selection against FHS requires identification of the birds with subclinical disease with biomarkers from a source such as blood. Prednisolone can induce femoral head problems and predisposition to FHS. Using this experimental model, we analyzed the plasma peptides and proteins from normal and FHS-prone chickens by mass spectrometry to identify differentially expressed peptides and proteins. We found two peptides, both derived from apolipoprotein A-I, quantitatively elevated and two proteins, protocadherin 15 and VEGF-C, that were conspicuously absent in FHS-susceptible birds.
Keywords: femoral head separation, glucocorticoids, chickens, mass spectrometry, biomarker, proteomics
Introduction
Femoral head separation (FHS) is an idiopathic bone disease of commercial poultry, which affects proximal femur leading to the separation of articular cartilage from its growth plate and renders the birds prone to bone infection, femoral head necrosis (FHN), and lameness.1–6 FHS occurs in rapidly growing broilers and breeders leading to production losses and welfare issues.7 Dystrophic and degenerative changes in the femoral epiphysis, most likely, predispose the articular and the growth plate cartilage to separate under minimal stress. Early identification of FHS-prone birds using biomarkers can facilitate their elimination from the breeding pool. However, the infrequent occurrence of FHS in a normal population of birds can be a limiting factor for its study that can be circumvented by the use of experimental models of the disease. Glucocorticoids induce avascular necrosis of femoral head in adult mammals and birds that can be the closest model for avian FHS.8–11 However, the early detection of FHS during its subclinical progression and its pathogenesis has not been addressed in younger animals including poultry. Previously we found that a synthetic glucocorticoid, prednisolone, was able to increase predisposition of chickens to FHS.12,13 Similarly, dexamethasone, another synthetic glucocorticoid, was reported to induce lameness in broilers that was attributed to femoral head problems.14 Serum or plasma metabolites and biomolecules can be a rich source of biomarkers because the disease-associated changes can lead to their qualitative and quantitative alterations. Since proteins constitute both structural and functional basis of the tissues, we hypothesized that changes in proteins may be useful as biomarkers. Therefore, we used plasma protein and peptides to find their changes under prednisolone-induced predisposition of young broilers to FHS.
Methods
Animals
The animal procedures were approved and carried out in accordance with the University of Arkansas IACUC guidelines. Forty eight Cobb 500 broiler chickens were raised on floor pens at the density of 8 square feet/bird from day 1 through 39, provided diets formulated as per National Research Council specifications15 and ad libitum water. The birds were divided into two groups: one received saline and the other prednisolone (MP Biomedicals) suspended in saline, administered by gavage at an approximate dose of 10 mg/kg body weight (BW) on days 28 and 34. On day 39, the chickens were bled through the wing vein and blood was collected in K-EDTA Vacutainer tubes (BD Bioscience), and then the chickens were euthanized. At necropsy, the femoral joints were subjected to a mild dorsal pressure at the hip joint to induce FHS.12,13 Chickens with predisposition to FHS showed the separation of articular cartilage from its growth plate with mild to severe damage, whereas the healthy femurs remained intact. Femoral heads from five birds in each group were fixed in formalin for histology. The sample sizes for the analytical methods are listed in Table 1.
Table 1.
Sample size for analytical methods.
| METHODOLOGY | NUMBER OF SAMPLES IN A GROUP |
|---|---|
| Animal experiment | 48 |
| Clinical chemistry | 9–11 |
| Histology | 5 |
| Peptide analysis | 3 pooled samples (each pool is made by mixing equal volumes of plasma from 3 birds) |
| Proteomic analysis | 2 pooled samples prepared as above |
Clinical chemistry and histology
Blood was centrifuged at 2,000 g for 10 minutes to separate plasma and stored at −20 °C for subsequent clinical chemistry and proteomic analyses. Only the plasma samples from normal chickens with intact femoral heads (CTRL) and those predisposed to FHS induced by prednisolone (FHS) were used for analyses. Albumin, cholesterol (CH), triglycerides (TG), and high density lipoprotein (HDL) concentrations in plasma were analyzed using an Express plus automated clinical chemistry analyzer (Ciba-Corning Diagnostics Corp). Low density lipoprotein (LDL) concentrations were calculated using the following formula: LDL = TC − HDL − TG/5.0 (mg/dL).16 The femoral head tissues were embedded in paraffin and processed for histology. Hematoxylin–eosin stained sections were examined and photographed using an Olympus IX-70 microscope. BW, FHS incidences, and serum chemistry were analyzed by a GLM procedure with pooled standard error of mean and significant means differentiated using Duncan’s multiple range tests using SAS software.17 Means were considered significant at P ≤ 0.05.
Plasma peptide and protein analysis
For peptide analyses, we used three samples from each of the CTRL and FHS groups, with each sample prepared by pooling equal volumes of plasma from three individual birds. An aliquot of plasma sample was mixed with two volumes of acetonitrile (ACN) containing 0.1% formic acid (FA) and kept at −20 °C for 12 hours to precipitate high abundance proteins.18,19 The precipitates were centrifuged at 10,000 g for 15 minutes at 4 °C, and the high abundance protein depleted (HAPD) supernatant was transferred to fresh tubes and dried using a CentriVap vacuum concentrator (Labconco). The dried content in each tube was dissolved with 0.1% FA to the original volume and desalted using reverse phase (RP) C18 Bond Elut tips (Agilent Technologies) as per manufacturer’s protocol with some minor modifications, which consisted of the binding and washing steps repeated five times before final elution. For cation exchange separation, the dried ACN supernatants were separately dissolved in 25 mM sodium acetate buffer pH 5.5 and fractionated using mini SCX columns (Pierce). The eluted materials that contained 0.5 M NaCl were then desalted with Bond Elut C18 tips prior to subsequent steps.
MALDI analysis
The eluted samples from both procedures were spotted (1 μL per spot) on a MALDI 384 target dried and overlaid with an equal volume of sinapinic acid (10 mg/mL 0.1% FA in 50% ACN). The spots were analyzed using an Ultraflex II MALDI-TOF/TOF instrument (Bruker Daltonics) in positive ion linear mode. The instrument was calibrated using a 5–17.5 kDa protein standard (Bruker Daltonics), and the MS data for peptides between the 1–10 kDa range were collected in an automated mode using the Bruker Flex control software with a constant laser power and 800 laser shots per spot.
ClinProTools analysis
The MS spectra of peptides from both CTRL and FHS samples were compared using ClinProTools software™ (CPT, Version 2.2, Bruker Daltonics).20 The quick classifier algorithm was used for automatic peak detection and integration, using peaks exhibiting a signal-to-noise ratio ≥10 and a threshold intensity of at least 5% relative to the largest peak.21 Individual peaks from all six samples were aligned and their areas analyzed for statistical differences. Anderson–Darling test was used to establish the data distribution and the statistical differences calculated using t- and Wilcoxon tests, respectively. As CPT does not perform multiple testing correction (MTC), we considered the best discriminating peaks with P ≤ 0.05 in all of the three tests (PAD, PTTA, and PWKW) without any MTC. Although several peaks were different based on CPT analysis, only the peaks observed in reverse phase-high performance liquid chromotography-electrospray ionization-mass spectrometry (RP-HPLC-ESI-MS) were isolated by RP-HPLC.
RP-LC-ESI-MS
HAPD plasma samples of CTRL and FHS groups were dried, dissolved in 0.1% FA, and subjected to RP-HPLC using a Supelco C18 column (15 cm × 4.6 mm, 5 μm particle size, 300 Å pore size, Sigma-Aldrich) attached to a Hewlett 110 HPLC system. The fractions were separated at a solvent flow rate of 0.7 mL/minute using 0 to 100% gradient of 0.1% FA (solvent A) and ACN (solvent B) over a period of 150 minutes. The HPLC was coupled online to a quadrupole ion trap ESI mass spectrometer (ESI-MS; Bruker Esquire 2000, Bruker) operated in positive ion mode with a dry gas temperature of 300 °C and flow of 12 mL/minute, and a nebulizing N2 pressure of 2.1 × 105 kPa. The mass spectrometer was optimized at m/z 1000 with low skimmer voltage to avoid ion fragmentation and charge stripping. The fractions corresponding to differentially expressed peptides determined by ClinproTools analyses were collected in several runs, pooled, dried, and reconstituted with 50 mM ammonium bicarbonate prior to further processing for their identification.
MALDI peptide mass fingerprinting for LC fractions
The pooled fractions of peptides were reduced with 10 mM dithiothreitol (DTT) for 1 hour at 60 °C and alkylated with 55 mM iodoacetamide (MP Biomedicals) for 1 hour in the dark at room temperature. Excess iodoacetamide was neutralized with DTT, and then the peptides were digested with trypsin (Promega) at 37 °C for 16 hours. The tryptic digests were desalted with Bond Elut C18 tips and spotted on a MALDI target plate with an equal volume of α-cyano-4-hydroxycinnamic acid (HCCA) matrix (10 mg/mL of 50% ACN containing 0.1% FA). Mass spectra were obtained in reflector positive ion mode using a Bruker Daltonics Ultraflex II MALDI-TOF/TOF mass spectrometer. The MALDI peptide mass fingerprint (PMF) was subjected to tandem MS/MS using MALDI LIFT-TOF/TOF (Bruker Daltonics). Bruker Biotools 3.1 was used to combine PMF and LIFT-MS/MS data and searched with parameters listed below.
LC-MS/MS
Two samples of HAPD plasma from the control and FHS groups were dried with CentriVap concentrator, reconstituted with 50 mM ammonium bicarbonate to 10th volume of starting HAPD plasma, and the protein content of the solutions was estimated using the micro BCA method (Pierce). One hundred micrograms of protein from two samples per group were reduced and alkylated as described earlier, digested with 2 μg of trypsin at 37 °C for 48 hours, and centrifuged at 21,000 g for 10 minutes to remove any insoluble materials. The supernatant was subjected to LC-MS/MS using an Agilent 1200 series capillary C18 RP-HPLC coupled to a Bruker Amazon-SL quadrupole ion trap mass spectrometer, capable of performing data-dependent acquisition. Tryptic peptides were separated by reverse-phase liquid chromatography (RP-HPLC) using a Zorbax SB C18 column (150 × 0.3 mm, 3.5 μm particle size, 300 Å pore size, Agilent Technologies), with a solvent flow rate of 6 μL/minute, and a gradient of 0 to 40% consisting of 0.1% FA (solvent A) and ACN (solvent B).
Data analysis
The peaks with intensities ≥10,000 counts and S/N >5 in LC-MS/MS chromatogram were used to obtain MS/MS peak lists and perform database search. The Proteinscape™ bioinformatics suite from Bruker Daltonics, coupled with the MASCOT 2.1 search engine (Matrix Science), was used to identify peptides in the NCBI Gallus gallus protein database with following parameters: single miscleavage, fixed carbamidomethylation of cysteine, variable methionine oxidation, and parent ion mass tolerance and fragment ion mass tolerance of 0.6 Da. Peptides with fragmentation ion score of 10 or higher were considered for protein identification. MASCOT automatic decoy database search was also performed with LC-MS/MS datasets. Proteins with <1% false discovery rate (FDR) with at least one unique peptide and a MASCOT score of ≥45 were reported. Common proteins from two samples in each of the CTRL and FHS groups were selected with online software (http://www.xlcomparator. net). The proteins present in each of the two CTRL and FHS samples were tallied to find all expressed common proteins in both groups, which were then matched to find differentially expressed proteins in each group. Gene Ontology (GO) annotations of the proteins were done using the DAVID bioinformatics software (http://david.abcc.ncifcrf.gov/).22
Results
BW, serum chemistry, and histology
Prednisolone treatment reduced the BW of chickens compared with saline (1.63 ± 0.22 kg vs 2.10 ± 0.14 kg, P ≤ 0.05, n = 24) and increased the FHS incidence by 38%. The plasma levels of albumin, CH, HDL, and LDL were significantly higher in prednisolone-treated birds but the TG concentrations were not statistically different (Table 2). Histology of femoral head segments of the prednisolone-treated birds showed increased adipogenesis (Fig. 1).
Table 2.
Effect of prednisolone on plasma albumin and lipids.
| PLASMA VARIABLES | CTRL (N = 11) | FHS (N = 9) |
|---|---|---|
| Albumin (mg/dL) | 1.31 ± 0.0a | 1.5 ± 0.0b |
| Cholesterol (mg/dL) | 122.9 ± 3.5a | 138.2 ± 3.9b |
| High density lipoprotein (HDL) (mg/dL) | 36.6 ± 1.1a | 42.2 ± 1.2b |
| Low density lipoprotein (LDL) (mg/dL) | 80.8 ± 2.5a | 89.5 ± 2.6b |
| Triglycerides (mg/dL) | 27.0 ± 0.5a | 32.4 ± 3.2a |
Notes: Values are reported as means ± SEM. Values with different superscripts indicate P ≤ 0.05.
Figure 1.
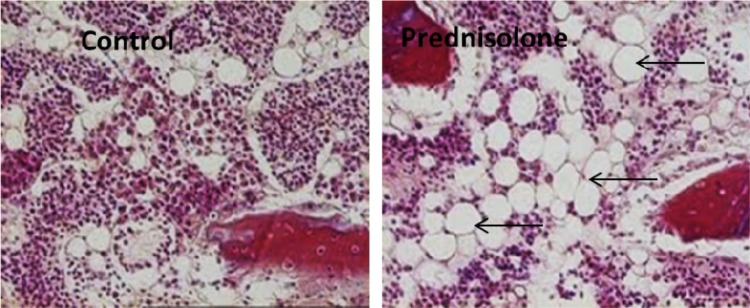
Histology showing prednisolone-induced bone marrow adipogenesis (arrows indicate adipocytes).
CPT analysis and the identification of peptides
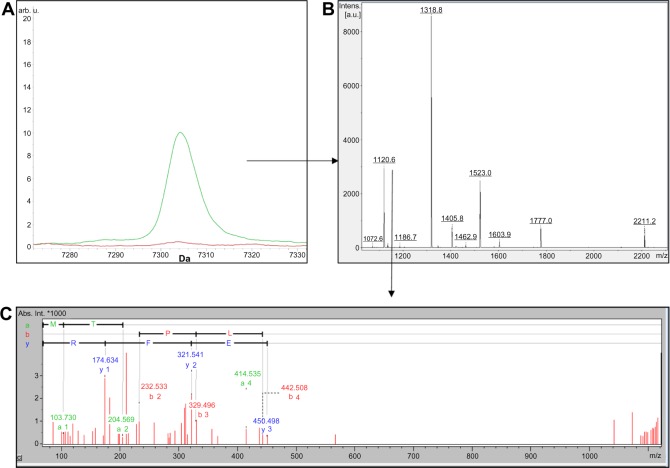
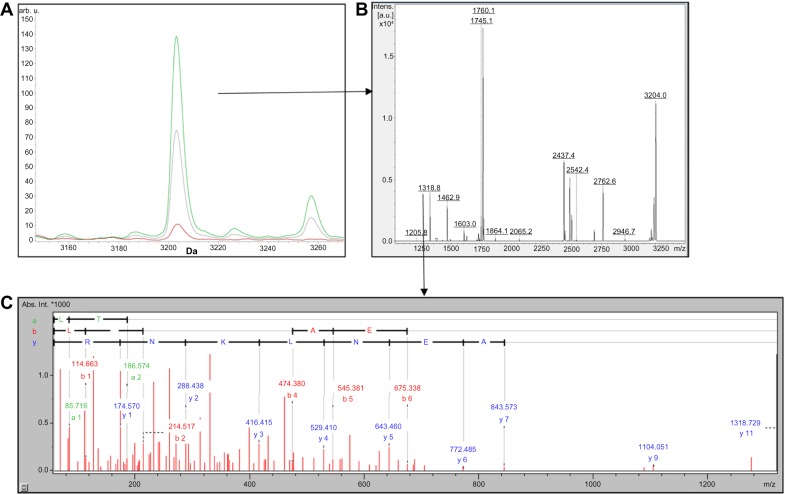
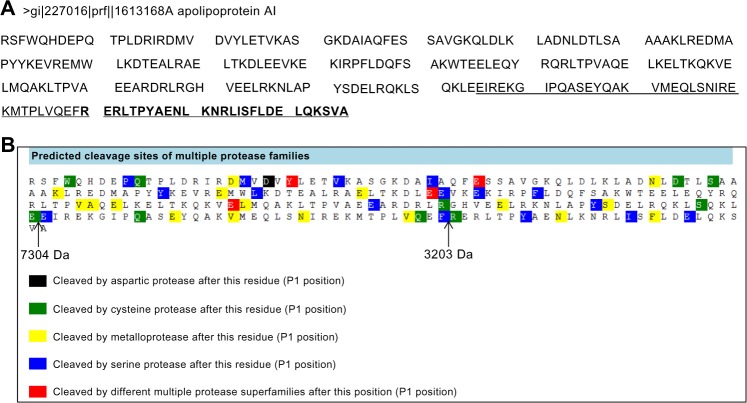
The peptide profiles of CTRL and FHS samples obtained by RP and SCX fractionation methods are shown in Supplementary Tables S1 and S2. Although several peaks between the two groups were different as per CPT analysis, we isolated only two peptides m/z 7304 and m/z 3203 (Figs. 2A and 3A) by RP-HPLC both of which were the fragments of chicken apolipoprotein A-I (APOA1) derived from its C-terminal region. The peptide m/z 3203 was internal to m/z 7304 sequence as shown by MS and MS/MS results (Figs. 2B, 2C, 3B, and 3C). In silico analysis using PROSPER23 suggested a probability of the generation of these fragments by the action of cysteine and serine proteases, respectively (Figs. 4A and 4B).
Figure 2.
(A) Comparison of MADLI-TOF mass spectra of C18 fractionated HAPD plasma showing the m/z 7304 peak analyzed by ClinproTools. The average spectra from CTRL (red) and FHS (green) groups represent the cumulative results of three pooled samples from nine birds. (B) Peptide mass fingerprint (PMF) of 7304 Da peptide. (C) Tandem mass spectrometry (MS/MS) of 1120 Da tryptic fragment derived from 7304 Da peptide.
Figure 3.
(A) Comparison of MADLI-TOF mass spectra of SCX fractionated HAPD plasma showing m/z 3203 region analyzed by ClinproTools. The average spectra from CTRL (red) and FHS (green) groups represent the cumulative results of three pooled samples from nine birds. (B) Peptide mass fingerprint (PMF) of 3203 Da peptide. (C) Tandem mass spectrometry (MS/MS) of 1318 Da tryptic fragment derived from 3203 Da peptide.
Figure 4.
(A) Protein sequence of chicken apolipoprotein A-I and the corresponding regions representing the 7304 Da (underlined) and the 3203 Da peptide (shown in bold) and (B) the possible cleavage sites that may generate these peptides, predicted by PROSPER online software.
LC-MS/MS proteomics
The lists of proteins identified in each of the two pools of CTRL and FHS samples are provided in Supplementary Tables S3, S4, S5, and S6, respectively. Among these identifications, less than a quarter (~22%) were annotated while the rest belonged to the predicted (~73%) and hypothetical proteins (~5%). A qualitative comparison of proteins expressed in CTRL and FHS groups was done to identify common and differentially expressed proteins in each group. Comparison of proteins using GO annotations showed that protocadherin-15, a protein associated with adult walking behavior, and vascular endothelial growth factor-C (VEGF-C), responsible for angiogenesis, and some calcium ion binding proteins were absent in the FHS samples (Table 3).
Table 3.
List of differentially expressed proteins and their relevant GO annotations.
| PROTEIN | CTRL | FHS | SIGNIFICANCE FROM GO ANNOTATION |
|---|---|---|---|
| Protocadherin 15 | + | − | Adult walking behavior and calcium ion binding |
| Similar to myosin-9 | + | − | Blood vessel development |
| Vascular endothelial growth factor C (VEGF) isoform-2 | + | − | Growth factor activity |
| Aczonin | + | − | Calcium ion binding |
| Mitogen-activated protein kinase kinase kinase 1 | + | − | Ubiquitin mediated proteolysis |
| Cullin 2 | − | + | Ubiquitin mediated proteolysis |
| Thyroid hormone receptor interactor 12 | − | + | Ubiquitin mediated proteolysis |
| Zinc finger homeodomain 4 | + | − | Regulation of transcription |
| SET domain containing 1B | + | − | Regulation of transcription |
| Prohibitin 2 | − | + | Regulation of transcription |
| Zinc finger homeobox 3 | − | + | Regulation of transcription |
| Telomeric repeat binding factor (NIMA-interacting) 1 | − | + | Regulation of transcription |
Discussion
Glucocorticoids at pharmacological concentrations exert both anti-anabolic and catabolic effects on skeletal tissues.24–26 In younger animals such as 4- to 6-week-old birds, the anti-anabolic effects may be the principal mechanism that causes the shrinkage and the arrest of growth plate development, which could lead to FHS.13 The glucocorticoid-induced dyslipidemia and bone marrow hyper-adipogenesis noted in our studies have also been reported by other investigators.27,28 However, the objective of the current study was to identify the changes in plasma proteins and peptides that may be relevant in glucocorticoid-induced FHS. Peptide and protein profiles were therefore compared to identify qualitative and quantitative differences in both groups.
We identified two peptides derived from the C-terminal region of APOA1, which is a major component of HDL as well as LDL, VLDL, and IDL.29–31 Prednisolone raises the blood levels of both HDL and LDL, which may undergo degradation affecting the levels of their peptide fragments. Thus, APOA1 peptide fragments can be formed (i) by random degradation of their parent proteins during extraction procedure or (ii) by the action of specific proteolytic enzyme(s). Because, in our experiment, both CTRL and FHS samples were extracted identically, the differential increase in APOA1 peptide levels in FHS samples, most probably, is related to the physiology of the birds rather than to the extraction procedures. Based on PROSPER analysis, it appears that both 7304 and 3203 Da fragments could be generated from APOA1 by the action of certain cysteine and serine proteases. Glucocorticoids, at high concentrations, induce apoptosis in many cells particularly the endothelial cells, which can generate microvascular problems and growth factor deficiencies.32,33 Apoptotic cell death accompanies the activation of endoproteases such as caspase.34,35 During endothelial cell apoptosis, these cysteine proteases can degrade HDL generating the APOA1 peptide fragments. However, the mechanism for the generation of APOA1 peptide fragments is not clear. Nonetheless, the glucocorticoid-induced hyperlipidemia and thromboembolism or endothelial apoptosis raise the possibility of vascular and nutritional deprivation in proximal femur. Because, the avian growth plate is relatively more vascular than its mammalian counterpart,36 the integrity of epiphyseal growth plate may be affected because of growth factor deprivation leading to its separation from articular cartilage.
Comparison of proteomic data of CTRL and FHS groups showed that almost a third of total proteins are common to both while the remaining were group specific. We presume that the proteins present only in CTRL samples are associated with healthy stage because they were absent in the FHS group, and those identified only in the FHS group may be associated with the disease. Analyzing these differentially expressed proteins with DAVID showed that the proteins associated with GO, such as angiogenesis, ubiquitin-mediated proteolysis, calcium binding, transcription factors, and adult walking behavior, were different in the FHS group. The proteins reported here, however, were selected based on one of the two criteria: (1) GO was totally absent in FSH but present in the CTRL (eg: VEGF) and (2) the same GO was present in both groups but the proteins classified under that GO were different (eg, some ubiquitin-related proteolysis and transcription factors). These differences might be associated with the mechanisms for FHS susceptibility in prednisolone-treated birds.
Protocadherin 15 (PCDH15), a protein associated with adult walking behavior,37 and VEGF-C isoform 2,38,39 a protein associated with blood vessel development, were conspicuously absent in the FHS group. PCDH15 belongs to the cadherin family, which are calcium-dependent cell adhesion proteins that are involved in cell signaling and mechanotransduction.40 The impairment of adhesion can increase the vulnerability of growth plate to detach from its articular cartilage. It may also impair signal transduction mechanisms involved in the joint function. Similarly, the absence of angiogenesis-associated proteins VEGF-C isoform 2 and myosin-9 can contribute to “avascular” conditions, which may predispose the birds to FHS.
The GO ubiquitin-mediated proteolysis was present in both CTRL and FHS samples. But the proteins cullin 241 and thyroid hormone receptor interactor 12 (TRIP-12)42 were present only in FHS samples. By contrast, the CTRL contained a different protein, namely, mitogen-activated protein kinase kinase kinase 1, which is also classified under the same GO, ubiquitin-mediated proteolysis (http://www.genome.jp/kegg-bin/show_pathway?map04120).43 Although direct experimental evidence to correlate these proteins and FHS was not found, they may be involved in the susceptibility to FHS and healthy conditions, respectively. Both CTRL and FHS samples showed the presence of different transcription factors in plasma, among which only prohibitin 2 was linked to stress44 while others were not characterized in relation to FHS. However, the significance of these differentially expressed proteins and their association with FHS or glucocorticoid-induced changes remain to be understood.
In conclusion, our results suggest that prednisolone-induced dyslipidemia and deficiencies of growth and adhesion factors may cumulatively contribute to the femoral head problems resulting in FHS. Plasma APOA1 and its degradation products may be useful as biomarkers for FHS-susceptible birds. Considering the limitation of these studies, which were performed in the glucocorticoid model, and idiopathic nature of the disease, these markers and association of APOA1, adipogenesis, and adhesion molecules must be verified in future using traditional approaches.
Supplementary Data
Table S1. List of peptide peaks different in the CTRL and FHS groups shown by Clinpro tools software (C18)
Table S2. List of peptide peaks different in the CTRL and FHS groups shown by Clinpro tools software (SCX)
Table S3. List of proteins identified in CTRL (pool 1)
Table S4. List of proteins identified in CTRL (pool 2)
Table S5. List of proteins identified in FHS (Pool 1)
Table S6. List of proteins identified in FHS (Pool 2)
Acknowledgments
We thank Scott Zornes, Sonia Tsai, and Wally McDonner for assistance. We thank David Cross for histology.
Glossary
List of Abbreviations
- LC-MS/MS
Liquid chromatography and tandem mass spectrometry
- ACN
Acetonitrile
- CH
Cholesterol
- CPT
ClinPro tools software
- FHN
Femoral head necrosis
- FHS
Femoral head separation
- GO
Gene Ontology
- HCCA
α-Cyano-4-hydroxycinnamic acid
- HDL
High density lipoprotein
- FA
Formic acid
- IDL
Intermediate density lipoproteins
- LDL
Low density lipoprotein
- MALDI-TOF-MS
Matrix assisted laser desorption ionization-time of flight-mass spectrometry
- MTC
multiple testing correction
- RP-HPLC-ESI-MS
Reverse phase-high pressure liquid chromatography-electrospray ionization-mass spectrometry
- SCX
Strong cation exchange
- TG
Triglycerides
- TRIP-12
Thyroid hormone receptor interactor 12
- VEGF-C
Vascular endothelial growth factor isoform C
- VLDL
Very low density lipoprotein
Footnotes
Author Contributions
Conceived and designed the experiment: NCR, BP, RL. Analyzed the data: BP, RL. Wrote the first draft of the manuscript: BP, NCR. Contributed to the writing of manuscript: RL, RO, JL. Agree with results and conclusion: NCR, RO, JL. Jointly developed the structure and argument of the paper: BP, NCR, RL, JL. Made critical revision and final approval of the manuscript: NCR, RL, JL. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Karen Pulford, Associate Editor
FUNDING: This study was funded by a grant from Cobb-Vantress Inc., and part of the study was carried out in the Statewide Mass Spectrometry Facility, supported by a NIH grant P30 GM103450 to the University of Arkansas. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
DISCLAIMER: Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA and does not imply its approval to the exclusion of other products that may be suitable. USDA is an equal opportunity provider and employer.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Julian RJ. Production and growth related disorders and other metabolic diseases of poultry – a review. Vet J. 2005;169:350–69. doi: 10.1016/j.tvjl.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Dinev I. Clinical and morphological investigations on the prevalence of lameness associated with femoral head necrosis in broilers. Br Poult Sci. 2009;50:284–90. doi: 10.1080/00071660902942783. [DOI] [PubMed] [Google Scholar]
- 3.Olkowski AA, Laarveld B, Wojnarowicz C, et al. Biochemical and physiological weaknesses associated with the pathogenesis of femoral bone degeneration in broiler chickens. Avian Pathol. 2011;40:639–50. doi: 10.1080/03079457.2011.626017. [DOI] [PubMed] [Google Scholar]
- 4.Bradshaw R, Kirkden R, Broom D. A review of the aetiology and pathology of leg weakness in broilers in relation to welfare. Avian Poultry Biol Rev. 2002;13:45–103. [Google Scholar]
- 5.McNamee PT, Smyth JA. Bacterial chondronecrosis with osteomyelitis (‘femoral head necrosis’) of broiler chickens: a review. Avian Pathol. 2000;29:477–95. doi: 10.1080/030794500750047243. [DOI] [PubMed] [Google Scholar]
- 6.Thorp BH, Whitehead CC, Dick L, Bradbury JM, Jones RC, Wood A. Proximal femoral degeneration in growing broiler fowl. Avian Pathol. 1993;22:325–42. doi: 10.1080/03079459308418924. [DOI] [PubMed] [Google Scholar]
- 7.Cook ME. Skeletal deformities and their causes: introduction. Poult Sci. 2000;79:982–4. doi: 10.1093/ps/79.7.982. [DOI] [PubMed] [Google Scholar]
- 8.Kerachian MA, Séguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114:121–8. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyanishi K, Yamamoto T, Irisa T, et al. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002;30:185–90. doi: 10.1016/s8756-3282(01)00663-9. [DOI] [PubMed] [Google Scholar]
- 10.Boss JH, Misselevich I. Osteonecrosis of the femoral head of laboratory animals: the lessons learned from a comparative study of osteonecrosis in man and experimental animals. Vet Pathol. 2003;40:345–54. doi: 10.1354/vp.40-4-345. [DOI] [PubMed] [Google Scholar]
- 11.Cui Q, Wang GJ, Su CC, Balian G. The Otto Aufranc award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997;344:8–19. [PubMed] [Google Scholar]
- 12.Durairaj V, Okimoto R, Rasaputra K, Clark FD, Rath NC. Histopathology and serum clinical chemistry evaluation of broilers with femoral head separation disorder. Avian Dis. 2009;53:21–5. doi: 10.1637/8367-051908-Reg.1. [DOI] [PubMed] [Google Scholar]
- 13.Durairaj V, Clark FD, Coon CC, et al. Effects of high fat diets or prednisolone treatment on femoral head separation in chickens. Br Poult Sci. 2012;53:198–203. doi: 10.1080/00071668.2012.675429. [DOI] [PubMed] [Google Scholar]
- 14.Wideman RF, Jr, Pevzner I. Dexamethasone triggers lameness associated with necrosis of the proximal tibial head and proximal femoral head in broilers. Poult Sci. 2012;91:2464–74. doi: 10.3382/ps.2012-02386. [DOI] [PubMed] [Google Scholar]
- 15.NRC . Nutrient Requirements of Poultry. Washington: National Academies Press; 1994. [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.SAS Institute . SAS/Stat User’s Guide, Version 8. Cary, NC: SAS Institute Inc; 2009. [Google Scholar]
- 18.Kay R, Barton C, Ratcliffe L, et al. Enrichment of low molecular weight serum proteins using acetonitrile precipitation for mass spectrometry based proteomic analysis. Rapid Commun Mass Spectrom. 2008;22:3255–60. doi: 10.1002/rcm.3729. [DOI] [PubMed] [Google Scholar]
- 19.Fernández C, Santos HM, Ruíz-Romero C, Blanco FJ, Capelo-Martínez JL. A comparison of depletion versus equalization for reducing high-abundance proteins in human serum. Electrophoresis. 2011;32:2966–74. doi: 10.1002/elps.201100183. [DOI] [PubMed] [Google Scholar]
- 20.Ketterlinus R, Hsieh SY, Teng SH, Lee H, Pusch W. Fishing for biomarkers: analyzing mass spectrometry data with the new ClinProTools software. Biotechniques. 2005;38(suppl 6):37–40. doi: 10.2144/05386su07. [DOI] [PubMed] [Google Scholar]
- 21.Bruker BioSciences Corp . Clinprotools 2.1 User Manual. Billerica: Bruker Bio-Sciences Corp; 2006. [Google Scholar]
- 22.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song J, Tan H, Perry AJ, et al. PROSPER: an integrated feature-based tool for predicting protease substrate cleavage sites. PLoS One. 2012;7:e50300. doi: 10.1371/journal.pone.0050300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bejar J, Peled E, Boss JH. Vasculature deprivation – induced osteonecrosis of the rat femoral head as a model for therapeutic trials. Theor Biol Med Model. 2005;2:24. doi: 10.1186/1742-4682-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lui JC, Baron J. Effects of glucocorticoids on the growth plate. Endocr Dev. 2011;20:187–93. doi: 10.1159/000321244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am. 2012;41:595–611. doi: 10.1016/j.ecl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang GJ, Cui Q, Balian G. The Nicolas Andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000;370:295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 28.Li SC, Lin CY, Kuo TF, et al. Chicken model of steroid-induced bone marrow adipogenesis using proteome analysis: a preliminary study. Proteome Sci. 2010;8:47. doi: 10.1186/1477-5956-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarugi P, Reggiani D, Ottaviani E, Ferrari S, Tiozzo R, Calandra S. Plasma lipoproteins, tissue cholesterol overload, and skeletal muscle apolipoprotein AI synthesis in the developing chick. J Lipid Res. 1989;30:9–22. [PubMed] [Google Scholar]
- 30.Hermann M, Foisner R, Schneider WJ, Ivessa NE. Regulation by estrogen of synthesis and secretion of apolipoprotein AI in the chicken hepatoma cell line, LMH-2A. Biochimica et Biophysica Acta (BBA)-Mol Cell Res. 2003;1641:25–33. doi: 10.1016/s0167-4889(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 31.Brewer HB, Fairwell T, LaRue A, Ronan R, Houser A, Bronzert TJ. The amino acid sequence of human APOA-I, an apolipoprotein isolated from high density lipoproteins. Biochem Biophys Res Commun. 1978;80:623–30. doi: 10.1016/0006-291x(78)91614-5. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the Hip 1. J Clin Endocrinol Metab. 2000;85:2907–12. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 33.Vogt CJ, Schmid Schönbein GW. Microvascular endothelial cell death and rarefaction in the glucocorticoid–induced hypertensive rat. Microcirculation. 2001;8:129–39. [PubMed] [Google Scholar]
- 34.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Wang X, Toney CB, Seamon J, Cui Q. Blood supply to the chicken femoral head. Comp Med. 2010;60:295–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed ZM, Riazuddin S, Bernstein SL, et al. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am J Hum Genet. 2001;69:25–34. doi: 10.1086/321277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vadasz Z, Misselevich I, Norman D, Peled E, Boss JH. Localization of vascular endothelial growth factor during the early reparative phase of the rats’ vessels deprivation-induced osteonecrosis of the femoral heads. Exp Mol Pathol. 2004;77:145–8. doi: 10.1016/j.yexmp.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Horowitz A, Seerapu HR. Regulation of VEGF signaling by membrane traffic. Cell Signal. 2012;24:1810–20. doi: 10.1016/j.cellsig.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–6. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber C, Dias-Santagata D, Glaser A, et al. Identification of mutations in CUL7 in 3-M syndrome. Nat Genet. 2005;37:1119–24. doi: 10.1038/ng1628. [DOI] [PubMed] [Google Scholar]
- 42.Poulsen EG, Steinhauer C, Lees M, Lauridsen A-M, Ellgaard L, Hartmann-Petersen R. HUWE1 and TRIP12 collaborate in degradation of ubiquitin-fusion proteins and misframed ubiquitin. PLoS One. 2012;7:e50548. doi: 10.1371/journal.pone.0050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 2014;42:D199–205. doi: 10.1093/nar/gkt1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu XH, Qian LJ, Gong JB, Shen J, Zhang XM, Qian XH. Proteomic analysis of mitochondrial proteins in cardiomyocytes from chronic stressed rat. Proteomics. 2004;4:3167–76. doi: 10.1002/pmic.200300845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of peptide peaks different in the CTRL and FHS groups shown by Clinpro tools software (C18)
Table S2. List of peptide peaks different in the CTRL and FHS groups shown by Clinpro tools software (SCX)
Table S3. List of proteins identified in CTRL (pool 1)
Table S4. List of proteins identified in CTRL (pool 2)
Table S5. List of proteins identified in FHS (Pool 1)
Table S6. List of proteins identified in FHS (Pool 2)