A 69-year-old Caucasian woman was brought to the emergency room (ER) from a skilled nursing facility where nursing staff had noticed jerking movements of her arms and legs. These movements began about 2 months previously, involved all four extremities and had worsened in the last 5 days. Her past medical history was significant for Wegener's vasculitis, end stage renal disease on hemodialysis, paroxysmal atrial fibrillation, hypertension and obstructive sleep apnea. Her medications included aspirin, warfarin, gabapentin, amiodarone (200 mg/day), midodrine (with hemodialysis), prednisolone, metoprolol succinate, citalopram, alprazolam, oxycodone-acetaminophen, fluticasone, loratadine and a Lidoderm patch. Her family history was negative for any neurological diseases, coronary artery disease or cancers. She had been staying at the nursing home for the last 2 months as part of rehabilitation after she had fallen and sustained fractures of her ribs and compression fractures of thoracic vertebrae. At the time of presentation, the patient was afebrile (36.7°C), normotensive (110/53 mm Hg), in normal sinus rhythm (60/min) and borderline tachypneic (20/min) saturating 95% on 3 liters of oxygen (baseline oxygen requirement). She was alert and oriented to time, place and person and verbalizing normally. She was noted to have asynchronous jerking movements involving her face and all four extremities. The patient denied any fevers, chills, nausea, abdominal pain, dysuria or diarrhea. The patient was admitted to the medicine floor for investigation of myoclonic jerks. About 3 h later, the patient was noted to be stuporous and non-verbalizing. The jerking movements were noted to be continuing. She was noted to be hypothermic (oral temperature 35.7°C), in sinus bradycardia (heart rate 46/min), mildly hypertensive (blood pressure 144/61 mm Hg) and tachypneic (respiratory rate 20/min).
Initial investigations in the ER revealed leucocytosis (13.9 × 103/μl, normal 4.0–11.0 × 103/μl), anemia (11.1 g/dl, normal 11.0–15.0 g/dl) with macrocytosis (mean corpuscular volume 103.1 fl, normal 79.0–97.0 fl), mild hyponatremia (134 mEq/l, normal 136–145 mEq/l), hyperkalemia (6.9 mEq/l, normal 3.6–5.1 mEql/l) and uremia (urea nitrogen 59 mg/dl, normal 6–20 mg/dl, and creatinine 8.11 mg/dl, normal 0.44–1.03 mg/dl). Her baseline urea nitrogen and creatinine were 15 mg/dl and 3.35 mg/dl respectively. Urine analysis was positive for 10–50 bacteria, a moderate amount of leucocyte esterase, 0–4 white blood cells and no squamous cells/hpf. When the patient became stuporous on the floor, an arterial blood gas was obtained which revealed hypercarbic respiratory acidosis (pH 7.09, pO2 70 mm Hg, pCO2 87 mm Hg, bicarbonate 25 mmol/l) (Table I). Serum thyroid-stimulating hormone (TSH) was noted to be 77.25 μIU/ml (normal 0.4–5.0 μlU/ml), free T4 0.4 ng/ml (normal 0.6–1.5 ng/ml) and free T31.7 pg/ml (normal 2.5–3.9 pg/ml). A random serum cortisol was noted to be 4.5 μg/dl (normal 6.7–22.6 μg/dl). A chest X ray revealed cardiomegaly without evidence of congestive heart failure and also showed increased soft tissue deposition in the cervical region (Figure 1A). A computed tomography (CT) scan of the head without contrast did not show any hemorrhage, masses, fluid or midline shift that could explain this sudden deterioration of consciousness.
Table I.
Laboratory values at presentation and during hospital stay
| Laboratory | At presentation | Transfer to ICU | At discharge | Reference range |
|---|---|---|---|---|
| WBC [× 103/μl] | 13.3 | 9 | 9.9 | 4.0–11.0 |
| Hgb [g/dl] | 11.1 | 9.4 | 11.2 | 11.0–15.1 |
| Hematocrit [g] | 36.6 | 30.1 | 36.1 | 33.1–44.5 |
| Platelet count [× 103] | 233 | 214 | 239 | 150–400 |
| MCV [fl] | 103.1 | 99.3 | 100.8 | 79.0–87.0 |
| MCHC [fl] | 30.3 | 31.2 | 31 | 32.0–36.0 |
| Differential count: | ||||
| Neutrophil [%] | 84 | 83 | 75 | 34–71 |
| Lymphocyte [%] | 8 | 8 | 14 | 15–51 |
| Monocyte [%] | 8 | 4 | 10 | 3–15 |
| Eosinophil [%] | 0 | 1 | 1 | 0–7 |
| Basophil [%] | 0 | 0 | 0 | 0–2 |
| Sodium [mEq/l] | 134 | 132 | 136 | 136–145 |
| Potassium [mEq/l] | 6.9 | 3.7 | 3.9 | 3.6–5.1 |
| Chloride [mmol/l] | 95 | 95 | 98 | 101–111 |
| Bicarbonate [mmol/l] | 26 | 28 | 31 | 22–32 |
| Urea nitrogen [mg/dl] | 59 | 40 | 37 | 6–20 |
| Creatinine [mg/dl] | 8.11 | 6.24 | 5.89 | 0.44–1.03 |
| AST | 15 | 17 | ||
| ALT | 19 | 17 | ||
| Alkaline phosphatase | 142 | 115 | ||
| Total bilirubin | 1.6 | 1.1 | ||
| C-reactive protein [mg/dl] | 5.5 | < 1.0 | ||
| Free T4 [ng/ml] | 0.4 | 0.8 | 0.6–1.5 | |
| Free T3 [pg/ml] | 1.7 | 1.5 | 2.5–3.9 | |
| TSH [μIU/ml] | 77.25 | 14.9 | 0.4–5.0 | |
| Random cortisol [μg/ml] | 4.5 | 6.7–22.6 | ||
| Arterial blood gas: | ||||
| pH | 7.09 | 7.35–7.45 | ||
| pO2 [mm Hg] | 70 | 80–100 | ||
| pCO2 [mm Hg] | 87 | 35–45 | ||
| Bicarbonate [mmol/l] | 25 | 18–26 | ||
| Base excess [mmol/l] | 3.4 | 2–3 | ||
| Urine analysis: | ||||
| Color | Yellow, clear | |||
| WBC [n/hpf] | 0–4 | < 5 | ||
| Bacteria [n/hpf] | 10–50 | 0–10 | ||
| Leucocyte esterase | Trace | Negative | ||
| Nitrite | Negative | Negative | ||
| Squamous cells [n/hpf] | 11-25 | 0–20 |
WBC – white blood cell count, MCV – mean corpuscular volume, MCHC – mean corpuscular hemoglobin concentration, AST – aspartate transaminase, ALT – alanine transaminase, TSH – thyroid-stimulating hormone.
Figure 1.
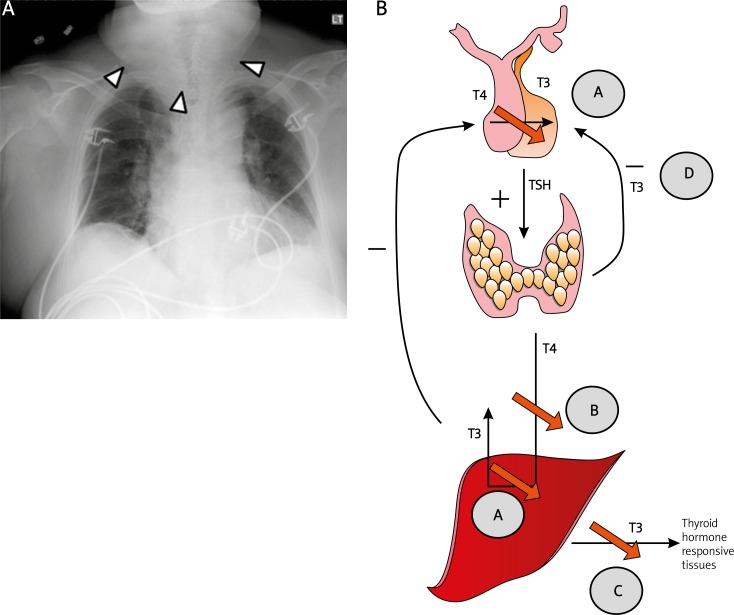
A – Comparison of chest X-rays at presentation with pre-treatment and last known normal chest X ray. All images were taken in postero-anterior orientation unless specified. (Left) At presentation, it revealed cardiomegaly without evidence of pleural effusion. Some soft tissue swelling is noticeable in the cervical region. (Middle) Chest X ray obtained prior to starting amiodarone reveals presence of cardiomegaly without the prominent neck soft tissue. (Right) Chest X ray taken four years previously reveals normal heart size and no soft tissue swelling. B – Mechanisms by which amiodarone inhibits thyroid function. Amiodarone inhibits (A) the peripheral conversion of T4 to T3 by inhibiting the enzyme 5'-deiodinase that converts T4 to T3 both within peripheral tissues and within the pituitary gland, (B) the entry of T4 into the peripheral tissues, and (C) the effect of T3 on thyroid-responsive tissues. T3 is the major negative regulator of TSH synthesis in the pituitary. The decrease in level of intra-pituitary T3 leads to increased synthesis of TSH seen in amiodarone-induced hypothyroidism
The differential diagnosis of myoclonic jerks includes physiological (associated with sleep or induced by anxiety, exercise or hiccups), physical encephalopathy (following hypoxia, trauma, heat stroke, electric shocks or decompression injury), focal damage to the central nervous system (following stroke, thalamotomy or due to a tumor, trauma, spinal cord or olivodentate lesions) epilepsy (usually seizures predominate), metabolic disturbances (hepatic or renal failure, hyponatremia, hypoglycemia, nonketotic hyperglycemia, multiple carboxylase deficiency), viral encephalopathies (herpes simplex or arbovirus encephalitis, subacute sclerosing panencephalitis and post-infectious encephalitis), toxins (bismuth, heavy metals, methyl bromide, drugs such as levodopa and tricyclic antidepressants), storage disorders (GM2 gangliosidosis, Tay-Sachs disease, Krabbe disease, Batten disease, Kuf's disease, Lafora body disease and sialidosis), spinocerebellar and basal ganglion degeneration, Huntington's, Parkinson's diseases, multiple system atrophy dementias (Alzheimer's and Creutz-feldt-Jakob disease), Hashimoto's encephalopathy, malabsorption syndromes, paraneoplastic syndromes and idiopathic.
The patient was intubated emergently and transferred to the ICU for mechanical ventilation.
In the ICU, the patient was given 200 μg of levo-thyroxine, 5 μg of liothyronine and 100 mg of hydrocortisone intravenously. She was also started on hydrocortisone therapy at 100 μg every 8 h intravenously.
Following initiation of the above treatment, the patient became progressively more alert and her jerking movements diminished. She was successfully extubated on the second day. She was switched first to 100 μg of levothyroxine intravenously once daily (on days 2 and 3) and then to 100 μg of levothyroxine orally daily (from day 4). The liothyronine (T3) dose was not repeated. The dose of hydrocortisone was decreased to 50 mg intravenously every 8 h (on day 3) and then to 25 mg intravenously every 8 h. Starting on day 4, she was switched to oral prednisone at a dose of 15 mg/day orally for 2 days and then a dose of 10 mg/day (her home dose). She underwent hemodialysis (days 3 and 5 after admission) without any complications. Her amiodarone which was held at admission was not restarted and she was discharged back to the skilled nursing facility in a stable condition. She remained in sinus rhythm during her entire hospital stay. On the day of discharge, her serum thyroxine levels were normal (0.8 ng/ml), T3 was unchanged (1.5 pg/ml) and TSH had begun to decrease (14.9 μIU/ml). A review of her records revealed that she had been started on amiodarone for control of paroxysmal atrial fibrillation 7 months previously. Thyroid function tests prior to starting therapy were normal (free T4 0.9 ng/ml and TSH 2.09 μIU/ml).
Myxedema coma is a syndrome caused by severe deficiency of thyroid hormone. Amiodarone, an iodinated derivative of benzofuran which bears structural similarity to the thyroid hormones tri-iodothyronine (T3) and tetra-iodothyronine (thyroxine or T4), can cause thyroid hypo- or hyperfunction. However, myxedema from amiodarone therapy is quite rare. The earliest reported cases of amiodarone-associated myxedema were by Guinet, who reported two cases in 1971 and 1972 [1, 2]. Grand then reported another case in 1975 [3]. Mazonson in 1984 described a case of a patient who was on high-dose (600 mg/day) amiodarone for 5 months and presented with lethargy, somnolescence, disorientation, hypotension and hypothermia. He was noted to have an elevated serum TSH (66 μU/ml, normal range 1–8 μU/ml) and a low T4 (0.8 μg/dl, normal range 5–12 μg/dl) and T3 (29 ng/ml, normal range 70–150 ng/dl) [4]. Since then, there has been only a handful of case reports of myxedema coma in patients treated with amiodarone (Table II) [5–7]. We present here a patient with this rare complication of amiodarone therapy.
Table II.
Summary of case reports of amiodarone-associated myxedema coma in the literature
| First author | Number ofcases | Age [years] | Gender | Indication for amiodarone | Duration of amiodarone therapy | Daily dose of amiodarone | Presenting symptoms | TSH | TPO antibodies | Management | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Khanderia | 1 | 72 | Male | Ventricular tachycardia | 3months | ND |
|
20μU/ml | ND | Levothyroxine | [5] |
| Raptis | 1 | 70 | Female | Atrial fibrillation | 2years | 200mg |
|
250mlU/l | 1:500 | Levothyroxine (100μg/kg) | [6] |
| Mazonson | 1 | 65 | Male | Ventricular tachycardia | 5months | 600mg |
|
65.9μU/ml | Negative | Levothyroxine andhydrocortisone ntravenously | [4] |
| Raeder | 2 | ND | ND | ND | ND | 9.7months (3–22) |
|
ND | ND | ND | [7] |
ND – not discussed, TPO – thyroid peroxidase.
The reported incidence of amiodarone-induced hypothyroidism (AIH) varies from 1% to 32% [8]. Female gender, older age, an underlying autoimmune thyroid disease, elevated baseline TSH levels, a starting dose of amiodarone > 200 mg/day, complex cyanotic heart disease and residence in an iodine sufficient region (e.g. United States) are considered to be risk factors for development of AIH [9]. Amiodarone is hypothesized to cause hypothyroidism by a) releasing iodine (during its metabolism), which exerts a negative feedback effect reducing the uptake of iodine by and synthesis of T3 and T4 by the thyroid gland, b) inhibiting the enzyme 5’-deiodinase, thus inhibiting conversion of T4 to T3, c) inhibiting uptake of T4 by peripheral tissues, and d) inhibiting the effect of T3 on thyroid receptors (Figure 1B). Myoclonic jerks are rare in myxedema and usually associated with Hashimoto's thyroiditis, which is diagnosed by positive thyroid peroxidase antibodies (not performed in our case), slowing of background EEG activity (present in our patient) and elevated protein and IgG in the CSF [10]. The patient did have a history of Wegener's, and a combination of the two autoimmune diseases has been reported rarely in the literature [11, 12]. That amiodarone potentiated an underlying subclinical autoimmune disease remains a possibility. In patients with central diabetes insipidus and other signs of hypopituitarism, involvement of the pituitary gland by Wegener's granulomatosis should be kept in mind [13, 14].
The management of myxedema coma involves replacement of the deficient hormone and supportive therapy. It is important to provide respiratory support by intubation, controlled mechanical ventilation and supplemental oxygen therapy as needed. Although hypothermic, external warming of the patient should be avoided as it can lead to peripheral vasodilatation and circulatory collapse. However, the patient can be covered with blankets at room temperature. Specific therapy consists of administering levothyroxine at a loading dose of 300–600 μg followed by a maintenance dose of 50–100 μg/day. Initial administration is preferably given intravenously as absorption from the gut is unpredictable. As the rate of conversion of T4 to T3 is decreased, supplemental T3 administration is suggested as a bolus of 10–20 μg followed by 10 μg intravenously every 4 h for the first 24 h, dropping to 10 μg every 6 h for days 2 and 3, after which oral administration can be resumed. Treatment of myxedema is associated with a risk of relative adrenal insufficiency secondary to enhanced peripheral metabolism of cortisol after T4 supplementation. Hence, glucocorticoid supplementation with hydrocortisone at a dose of 50–100 mg intravenously every 6–8 h is given for the first 7–10 days, after which it can be tapered and the further dose determined by the clinical response. Current evidence suggests that amiodarone can be continued in patients who receive concomitant levothyroxine supplementation. In those in whom stopping amiodarone therapy is appropriate, spontaneous remission of hypothyroidism usually occurs in 3–4 months.
In conclusion, we report a case of myxedema coma presenting as worsening myoclonic jerks in a previously euthyroid patient on amiodarone therapy. It emphasizes the importance of maintaining a high index of suspicion for this complication in patients with a history of amiodarone therapy.
References
- 1.Guinet P, Pousset G, Briere J, Grand Smith BG. Myxedema caused by iodine (apropos of a case due to amiodarone) Rev Fr Endocrinol Clin. 1972;13:229–43. [PubMed] [Google Scholar]
- 2.Guinet P, Pousset G, Briere J, Bianchi GG. Iodine myxedema during amiodarone treatment. Lyon Med. 1971;226:167–71. [PubMed] [Google Scholar]
- 3.Grand A. Myxedema caused by amiodarone: a further case. Coeur Med Interne. 1975;14:163–7. [PubMed] [Google Scholar]
- 4.Mazonson PD, Williams ML, Cantley LK, Dalldorf FG, Utiger RD, Foster JR. Myxedema coma during long-term amiodarone therapy. Am J Med. 1984;77:751–4. doi: 10.1016/0002-9343(84)90379-6. [DOI] [PubMed] [Google Scholar]
- 5.Khanderia U, Jaffe CA, Theisen V. Amiodarone-induced thyroid dysfunction. Clin Pharm. 1993;12:774–9. [PubMed] [Google Scholar]
- 6.Raptis L, Papathanasiou H, Pappas G, Akritidis N. It's all in the face: amiodarone-induced myxedema and skin pigmentation. Eur J Dermatol. 2006;16:590–1. [PubMed] [Google Scholar]
- 7.Raeder EA, Podrid PJ, Lown B. Side effects and complications of amiodarone therapy. Am Heart J. 1985;109:975–83. doi: 10.1016/0002-8703(85)90238-8. [DOI] [PubMed] [Google Scholar]
- 8.Harjai KJ, Licata AA. Effects of amiodarone on thyroid function. Ann Intern Med. 1997;126:63–73. doi: 10.7326/0003-4819-126-1-199701010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Padmanabhan H. Amiodarone and thyroid dysfunction. South Med J. 2010;103:922–30. doi: 10.1097/SMJ.0b013e3181e90500. [DOI] [PubMed] [Google Scholar]
- 10.Matsunaga A, Yoneda M. Anti-NAE autoantibodies and clinical spectrum in Hashimoto's encephalopathy. Rinsho Byori. 2009;57:271–8. [PubMed] [Google Scholar]
- 11.Armbruster C, Vetter N. Wegener's granulomatosis with splenic involvement and Hashimoto's lymphomatous thyroiditis. Pneumologie. 1991;45:28–31. [PubMed] [Google Scholar]
- 12.Masor JJ, Gal AA, LiVolsi VA. Hashimoto's thyroiditis associated with Wegener's granulomatosis. Am J Med Sci. 1994;308:112–4. doi: 10.1097/00000441-199408000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Santoro SG, Guida AH, Furioso AE, Glikman P, Rogozinski AS. Panhypopituitarism due to Wegener's granulomatosis. Arq Bras Endocrinol Metabol. 2011;55:481–5. doi: 10.1590/s0004-27302011000700008. [DOI] [PubMed] [Google Scholar]
- 14.Katzman GL, Langford CA, Sneller MC, Koby M, Patronas NJ. Pituitary involvement by Wegener's granulomatosis: a report of two cases. AJNR Am J Neuroradiol. 1999;20:519–23. [PMC free article] [PubMed] [Google Scholar]