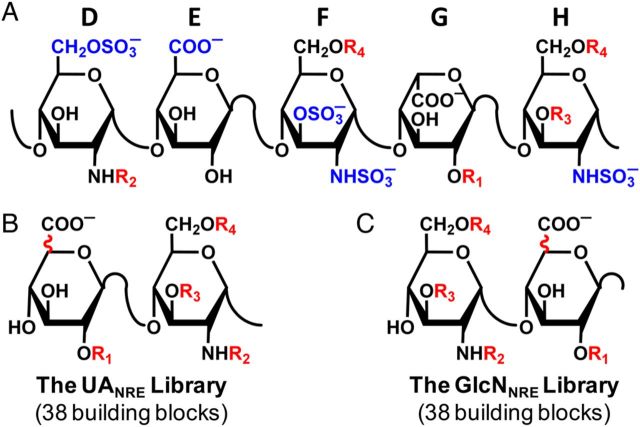
Fig. 1.
(A) Structure of natural pentasaccharide DEFGH, where D, E, F, G and H labels refer to historical assignment of residue labels (Desai et al. 1998). Residue D forms the non-reducing end, while H is at the reducing end of the polysaccharide chain. Groups highlighted in blue are critical for high-affinity interaction with antithrombin. R1, R2, R3 and R4 groups are variable groups. (B) The UANRE library of oligosaccharides (di- to octasaccharide) has a GlcAp or IdoAp residue at the non-reducing end and ends with a GlcNp residue at the reducing end. (C) The GlcNNRE library of oligosaccharides has a GlcNp residue at the non-reducing end and either a GlcAp or IdoAp residue at the reducing end. R1, R2, R3 and R4 variations, UAp epimerization variation and conformational variations (1C4 or 2SO) for IdoAp residue generate 38 disaccharide building blocks.