Table 1.
MurX-catalyzed syntheses of lipid I analogues from N-glycolyl- and N-acetyl-Park’s nucleotides
| |||||
|---|---|---|---|---|---|
|
| |||||
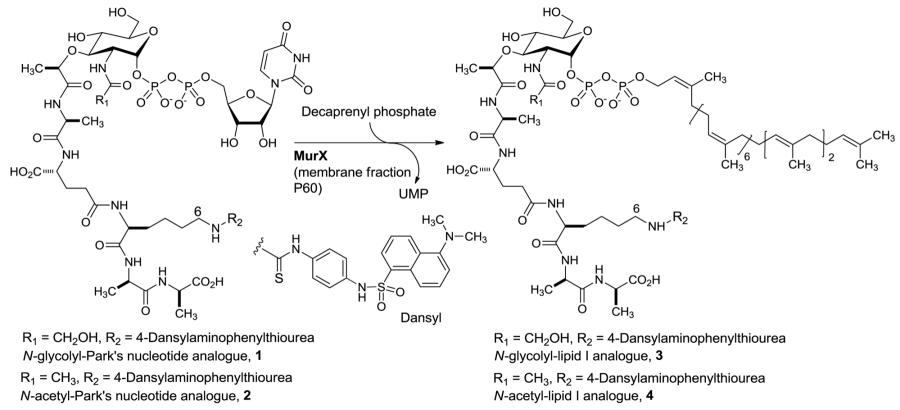
| Entry1 | Park’s nucleotide | Decaprenyl phosphate (equivalents against 1 or 2) |
Lipid I | Reaction time (h) |
Yield (%)2 |
| 1 | 1 | 2 | 3 | 1 | 15-20 |
| 2 | 1 | 2 | 3 | 3 | 15-20 |
| 3 | 1 | 10 | 3 | 1 | 20-25 |
| 4 | 2 | 2 | 4 | 1 | 15-20 |
| 5 | 2 | 2 | 4 | 3 | 15-25 |
| 6 | 2 | 10 | 4 | 1 | 20-25 |
Reaction conditions: Park’s nucleotide (2 mM; 3.75 μL), MgCl2 (0.5 M; 10 μL); KCl (2 M, 10 μL), Triton X100 (0.1%; 11.25 μl), Tris-buffer (pH = 8; 50mM, 5 μL), decaprenyl phosphate (10 mM, 2 or 10 equivalents against 1 or 2), P-60 (15 μL), 26 °C, 1 or 3h.;
n-butanol extract was analyzed by HPLC (column: Kinetex 5u C8 100A, 150×4.60 mm, solvent: CH3CN : 0.05 M aq. NH4HCO4, flow rate: 0.5 mL / min.
