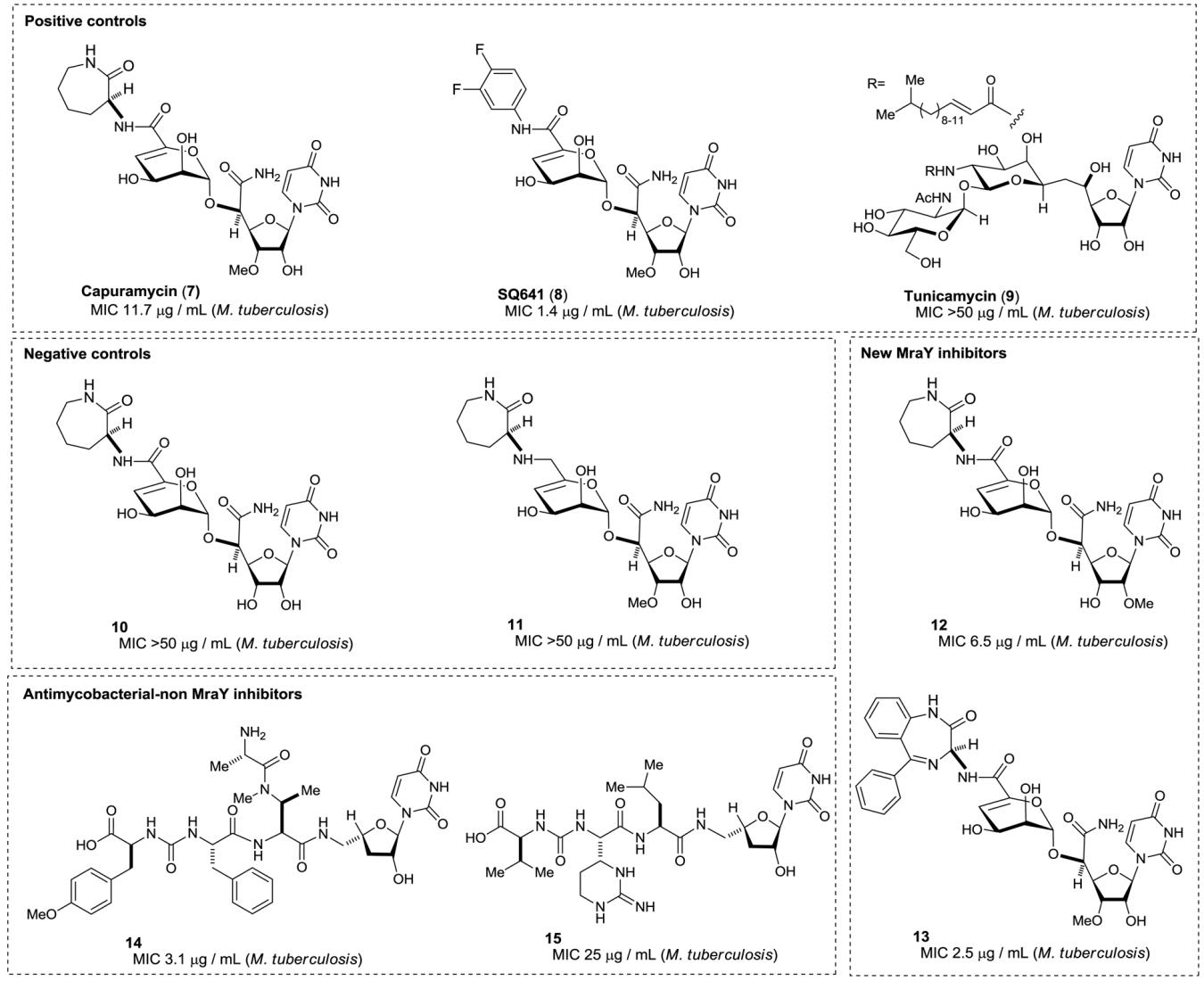
Table 2.
Assay of positive- and negative-controls, and a library of uridyl peptides against MurX
| ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| entry | Compound | MurX inhibition (%)a | IC50 (μM)b | |||||
|
|
||||||||
| 0.01 μM | 0.1 μM | 1 μM | 10 μM | 100 μM | ||||
| 1 | Capuramycin (7) | - | 36.6 | 88.4 | 100 | 100 | 0.152 ± 0.0125 | (0.150-0.0180)c |
| 2 | SQ641 (8) | - | 40 | 95 | 100 | - | 0.109 ± 0.00845 | (0.0150-0.100)c |
| 3 | Tunicamycin (9) | - | 9 | 42 | 72 | 72 | 2.73 ± 0.138 | (2.40-2.95)c |
| 4 | 10 | - | 0 | 0 | 0 | 0 | ND | |
| 5 | 11 | - | 0 | 0 | 0 | 0 | ND | |
| 6 | Vancomycin | - | 0 | 0 | 0 | 0 | ND | |
| 7 | 12 | 3.8 | 81.2 | 100 | 100 | - | 0.105 ± 0.00330 | |
| 8 | 13 | - | 40 | 95 | 100 | - | 0.0950 ± 0.00395 | |
| 9 | 14 | - | 0 | 0 | 0 | 0 | ND | |
| 10 | 15 | - | 0 | 0 | 0 | 0 | ND | |
| 11 | DMSO | 0 | 0 | 0 | 0 | 0 | ND | |
1) Reaction conditions: Park’s nucleotide-Nε-dansylthiourea 2 (75 μM; 3.75 μL), MgCl2 (0.5 M; 10 μL); KCl (2 M, 10 μL), Triton X100 (0.1%; 11.25 μl), Tris-buffer (pH = 8; 50 mM, 2.5 μL), neryl phosphate 6 (10 mM, 45 μL), inhibitor molecule (0-100 μM in DMSO (2.5 μL)), P-60 (15 μL), 26 °C, 3h.; The Km 18.29 μM (Park’s nucleotide 2).
The IC50 values were obtained three times and the standard error of the mean was calculated.
The IC50 values in parentheses were reported in the literatures (see, references 18, 19, 24, 25, 45) and/or were obtained via the assay conditions summazried in Table 1.; Reaction conditions: Park’s nucleotide-Nε-dansylthiourea 2 (75 μM; 3.75 μL), MgCl2 (0.5 M; 10 μL); KCl (2 M, 10 μL), Triton X100 (0.1%; 11.25 μl), Tris-buffer (pH = 8; 50 mM, 5 μL), decaprenyl phosphate (10 mM, 2 equivalents against 2), inhibitor molecule (0-100 μM in DMSO (2.5 μL)), P-60 (15 μL), 26 °C, 3h.; The Km 18.05 μM (Park’s nucleotide 2).
