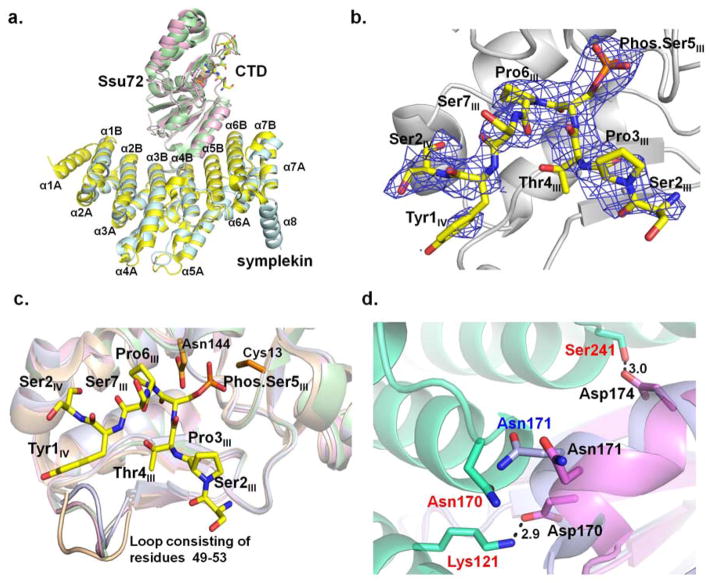
Figure 3.
Structure of Drosophila melanogaster Ssu72-symplekin-CTD ternary complex in which the ligand is 19-mer CTD peptide with singly phosphorylated Ser5:
a. The superimposed structures of Drosophila and human Ssu72-symplekin-CTD complexes. Symplekin and Ssu72 of Drosophila melanogaster are colored pale cyan and light pink, respectively. Symplekin and Ssu72 of human are colored yellow and green. CTD peptides bound by Drosophila and human Ssu72s were shown in stick model with the carbon atom colored yellow and green, respectively. b. Composite omit annealing map of electron density of CTD peptide of singly phosphorylated Ser5 (yellow) in Drosophila melanogaster Ssu72-symplekin complex, contoured at 3 σ. c. Overlay of the structures of Drosophila apo Ssu72 (wheat), Ssu72 in complex with the CTD (light pink), Ssu72 in complex with symplekin (grey), and Ssu72 in complex with symplekin and the CTD (green): a loop consisting of Ssu72 49–53 residues display a significant conformational change upon the binding of the CTD or symplekin. d. Interaction formed between Drosophila Ssu72 (violet) upon its association with template protein symplekin (lime green). Superimposition with apo Ssu72 (light blue) structure reveals a small conformational adjustment to accommodate symplekin binding.