Recently, the number of diabetic patients has increased very rapidly and is accompanied by an increasing development of diabetic neuropathies (DNs). The incidence and prevalence of DNs associated with duration of diabetes affects up to 50% of diabetic patients after 25 years of disease. Although DNs are known as the most common complications of diabetes, they was not adequately and properly diagnosed, and their severity is not estimated reliably in clinical practice by current methods. DNs have a wide variability in prevalence, from a few percentage points to over 50%. This could be explained by a lack of consensus about defining criteria; lack of sensitive, objective and quantitative diagnostic tools; and lack of homogeneity in research subjects of DNs. In addition, we do not have accurate and reproducible clinical end-point assessment modalities by each researcher. The importance of accurate and early detection of DNs is emphasized by the prediction of all-cause and disease-specific mortality in patients with diabetes accompanied with intensive glycemic control.
After the 1988 San Antonio conference on DNs, several diagnostic criteria for DNs were proposed, and recently, Tesfaye et al.1 proposed separate criteria for DNs. They proposed that DNs are symmetrical, length-dependent polyneuropathies attributable to metabolic and microvessel alterations as a result of chronic hyperglycemia exposure (diabetes) and cardiovascular risk covariates. Abnormalities in nerve conduction studies (NCS), which are frequently detected in subclinical DN conditions, are known to be the first objective quantitative indication of this condition. Furthermore, for research purposes, the authors suggested that confirmed and subclinical DNs must be evaluated by NCS.
If we suspect DNs in diabetic patients, to diagnose and characterize the condition we must exclude other causes of sensory motor neuropathies. Precise patient history and neurological examinations must be carried out to obtain much more information about the general characteristics. For reported symptoms and signs, and other clinical neurophysiological tests, such as quantitative sensory tests (QSTs), abnormal results were required to characterize the symptoms, signs and overall severity of the DNs. Because assessing methodologies and techniques, evaluation time and reference values, and validated QSTs are not sufficient for clinicians, the usual clinical evaluation and neurophysiological tests for DNs diagnosis and staging results have many limitations. Furthermore, the usual clinical evaluation and neurophysiological tests cannot define the nature of the pathophysiological changes and the clinical features that specify the distribution of nerve involvement or the time-course and stage. To accurately and reliably evaluate the kind, severity and distribution of sensation loss, we require more standardized, validated and referenced methods. Also, the clinician's proficiency has been added for adequate assessment of DNs by other researchers2.
As we know, DNs involve motor, sensory and autonomic nerves, and specify their symptoms and signs by damaged nerve fiber size and type. Therefore, variable parameters of damaged nerves and their manifestations are important for the diagnosis of DNs, in addition to a patient's neuropathic symptoms and signs. Because NCS investigate only large myelinated fibers, occasionally there is a discrepancy between the morphology and physiology of such small fiber neuropathy. Nerve fiber evaluation tests reflect three conditions: normal, axonal injury and myelin loss of peripheral nerves. Normal means that axons and myelinated fibers are intact, axonal injury shows that damaged axons disconnect fibers from sensory nerves or motor nerves. Finally, myelin loss occurs at multiple sites along the nerve, and results in the slowing of conduction velocities. Conduction velocity can be mildly slowed by metabolic causes, such as hyperglycemia, does not structurally affect myelin and is reversed by correction of underlying metabolic abnormalities. Parameters of NCS, such as peroneal conduction velocity and sural sensory nerve action potential (SNAP) amplitude expressed as percentiles and adjusted for variables of age and anthropomorphic variables, are especially sensitive indicators of DNs3. Various factors affect the rate of nerve conduction. Most important in NCS are the temperature of the tested nerve, normal variations among nerves and nerve segments, and patient age. NCS is slowed at lower temperatures in a linear manner, and the effects of temperature are more apparent with sensory than with motor nerves. With lower nerve temperatures, SNAPs are longer in duration, resulting in less phase cancellation and larger SNAP amplitudes. The lower nerve temperature slows conduction, by approximately 2 m/s/°C. To prevent misdiagnosis, the limb skin temperature of the patient should be maintained at over 31°C3.
In the clinically relevant late stage of DN complications, although the nerve parameter of NCS can use surrogate markers and widely accepted objective methods for the diagnosis of DNs, it cannot adequately evaluate the sensation loss area and severity. Therefore, the attributes of NCS are weak measures of DNs severity, and provide only limited information about the kind and distribution of sensory loss. Furthermore, NCS is a complex, time-consuming procedure, and requires specialized equipment and experts. Also, although abnormal parameters in NCS can predict the outcome of DNs, there is only limited data for the prediction of incipient DNs at a stage that precedes its complications. To evaluate the stage and severity of DNs, objective and/or quantitative measures, such as NCS and QSTs, are required. As the severity of DNs is a combination of neuropathic symptoms and signs, abnormal neurophysiological test results, and other neuropathic dysfunctions and impairments, the sum scores of various measures of neurological signs and symptoms, neurophysiological test scores, or scores of function of quality of life are require, and provide the grade of severity1.
Recently, some researchers reported that NCS could be used for the early detection and prediction of DNs. Hyllienmark et al.4 carried out a study to examine whether subclinical nerve dysfunction as reflected by electrodiagnostic testing predicts the development of clinical neuropathy in 59 type 1 diabetic patients who were aged 15.5 ± 3.22 years and duration of diabetes was 6.8 ± 3.3 years. At baseline, patients' nerve conduction velocities and amplitudes were modestly reduced without clinical neuropathy evidence compared with healthy controls. At follow up, approximately 13 years later, nine patients (15%) showed clinical neuropathy, and they showed more significant reductions in all tested nerve velocities and amplitudes, and showed a negative correlation between peroneal motor conduction velocity, sural sensory conduction velocity, sural SNAP, and peroneal compound muscle action potential and age. Also, patients' glycated hemoglobin was 6.9 ± 1.03% at baseline to 7.4 ± 0.94% at follow up, and was correlated with NCS results and neuropathy impairment assessment. From these results, they concluded that subclinical nerve dysfunction, as defined by NCS data, predicted clinical neuropathy many years later, and that the strongest predictor for the presence of clinical neuropathy after an average of 20 years with type 1 diabetes was poor metabolic control during the first years of the disease. Therefore, they emphasized that the role of early NCS and good metabolic control during the early years of type 1 diabetes was important to detect and predict DNs development. Although the role of early detection and prediction of NCS in DNs is useful, a limitation of that study was that the sample was composed of type 1 diabetes patients only. Therefore, further research into the early detection and predictive roles of NCSs as markers of nerve damage in type 2 diabetespatients is required. Weisman et al.5 carried out a different study in 406 participants (61 with type 1 diabetes and 345 with type 2 diabetes) to determine the measurement of single and simple combinations of NCS parameters for identification and future prediction of DNs. At baseline, 246 (60%) patients were prevalent cases, and after 4 years of follow up, 25 (23%) of the 109 prevalent controls that followed became incident DNs cases. From that study, they reported that threshold values for peroneal conduction velocity and sural SNAP best identified prevalent cases, and baseline tibial F-wave latency, peroneal conduction velocity and the sum of three lower limb nerve conduction velocities (sural, peroneal and tibial) best predicted 4-year incidence. Also, they concluded that individual NCS parameters or their simple combinations were valuable for identification and future prediction of DNs. However, that study required further study for the implication of the amplitude potential and conduction velocity threshold value that differ from the normal distributions of NCS parameters in detection and prediction of DNs.
In conclusion, because of the lack of unified diagnostic criteria for DN, we have many problems carrying out various clinical trials, such as therapeutic efficacy and epidemic research with unified research protocols. Therefore, DNs epidemic study results are variable by current diagnostic tools. To solve these problems, we required only one reasonable consensus definition for DNs diagnosis and prediction. Furthermore, each of the current diagnostic tools and procedures has their advantages and weakness. Both NCS and QSTs have high reproducibility and are complementary to each other. Neurological examinations, and neuropathic signs and symptoms are important in early detection and severity evaluation (Figure1). However, these assessments require good performance and proficiency of the physicians. For early detection and prediction of DNs, we must carry out several neurological examinations and NCSs in the lower extremities and both feet. It is well known that NCS changes in DNs patients occur earlier than clinical symptoms and signs without small fiber neuropathy, therefore NCS results are important in the early detection of DNs. Although current NCS performance has many limitations, proper nerve and machine selection for reproducible and convenient measurement is more important and valuable for early detection and prediction of DNs.
Figure 1.
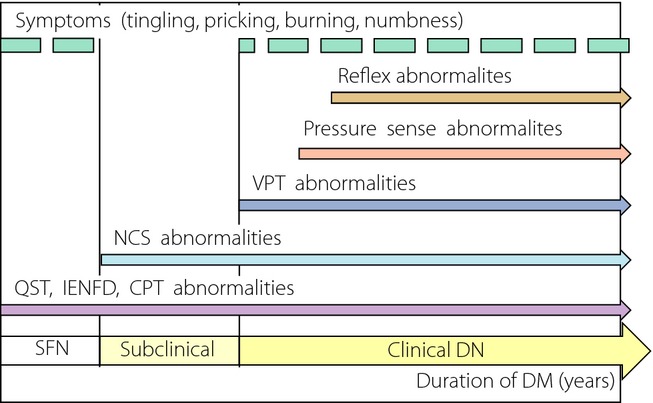
Abnormalities of diagnostic tool by diabetic peripheral neuropathy type. CPT, current perception threshold; DM, diabetes mellitus; DN, diabetic neuropathies; IENFD, intra-epidermal nerve fiber density; NCS, nerve conduction study; QST, quantitative sensory test; SFN, small fiber neuropathy; VPT, vibration perception threshold.
References
- Tesfaye S, Boulton AJ, Dyck PJ. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck PJ, Herrmann DN, Staff NP. Assessing decreased sensation and Increased sensory phenomena in diabetic polyneuropathies. Diabetes. 2013;62:3677–3686. doi: 10.2337/db13-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg MB. An electrodiagnostic approach to the evaluation of peripheral neuropathies. Phys Med Rehabil Clin N Am. 2013;24:153–168. doi: 10.1016/j.pmr.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Hyllienmark L, Alstrand N, Jonsson B. Early electrophysiological abnormalities and clinical neuropathy: a prospective study in patients with type 1 diabetes. Diabetes Care. 2013;36:3187–3194. doi: 10.2337/dc12-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman A, Bril V, Ngo M. Identification and prediction of diabetic sensorimotor polyneuropathy using individual and simple combinations of nerve conduction study parameters. PLoS One. 2013;8:e58783. doi: 10.1371/journal.pone.0058783. [DOI] [PMC free article] [PubMed] [Google Scholar]


