The average life expectancy of diabetic patients has been increasing year-by-year in Japan as a result of tremendous advances in medical technology, although it remains shorter than that of people without diabetes. However, their healthy life expectancy does not always keep pace. One of the most important reasons for healthy diabetic patients' life expectancy not being as long is thought to be the complication of dementia, and previous large-scale epidemiological studies have found that the incidence of dementia in diabetic patients is two- to threefold higher than in non-diabetic people. Therefore, prevention of dementia in an increasing population of elderly people with diabetes is an important issue in diabetes treatment.
Many underlying mechanisms of cognitive impairment have been studied, but they are still yet to be fully clarified. Up until now, those involving hyperglycemia, hypoglycemia and insulin disorders, such as insulin deficiency and insulin resistance, have been considered to be the main mechanisms1. First, hyperglycemia might be directly involved in nerve degeneration in the brain through advanced glycation end-products and oxidative stress, and vascular risk factors concomitant with hyperglycemia cause cerebrovascular disease, leading to cognitive dysfunction. Second, a consensus is being reached that severe hypoglycemia is associated with cognitive decline. Third, deficiency in insulin secretion impairs brain metabolism, resulting in a decline in cognitive functions, such as memory. Accumulation of amyloid-β peptide (Aβ) in the brain is the characteristic pathology for Alzheimer's disease, but insulin induces the release of Aβ to the brain exterior. Insulin also promotes the expression of insulin-degrading enzyme (IDE), which degrades Aβ. In contrast, in the case of hyperinsulinemia, IDE is consumed, resulting in accumulation of Aβ. In addition, insulin resistance in the brain has recently drawn considerable attention as a potent mechanism of Alzheimer's disease.
An association between diabetic microvascular disease (in particular retinopathy and nephropathy) and cognitive impairment has been reported. Therefore, evaluation of the retina and kidney could be a way to screen for an underlying vascular etiology in people with cognitive impairment. It is thought that this association is based on the same metabolic abnormality rather than cause and effect, as organs such as the retina and kidney and cerebral small vessels are considered to have a similar embryological origin and structures, and share common physiological characteristics. However, it cannot be said that an association between microvessel damage in the retina and kidney is necessarily confined to diabetics, though it is likely that hyperglycemia and insulin resistance in diabetic patients impair microvessels through endothelial dysfunction as a result of glycation, oxidative stress and increased activity of the polyol pathway as compared with non-diabetic people. In the brain, these factors might affect the incidence and progression of cerebral small vessel disease (SVD), such as asymptomatic brain infarction, white matter lesions and microbreeds, which are also reported to be associated with cognitive impairment, in addition to the incidence of ischemic stroke2. As a mechanism, it is thought that endothelial dysfunction in brain microvessels might cause disruption of the blood–brain barrier, leading to impaired Aβ clearance as well as incidence of SVD. Therefore, from the standpoint of diabetic vascular disease, endothelial dysfunction could play a central role in cognitive impairment through microvessel disease (Figure1).
Figure 1.
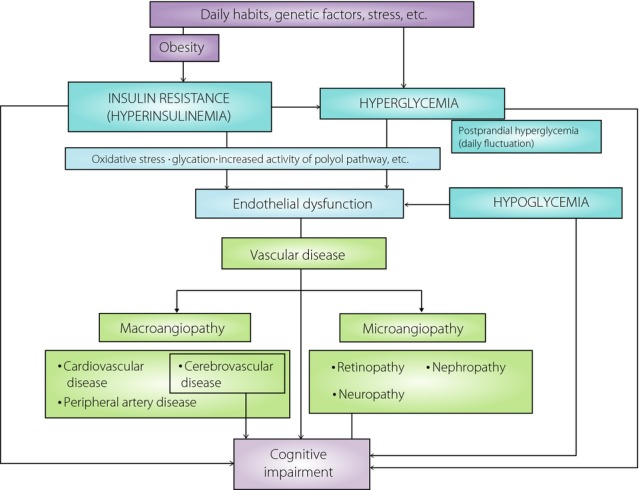
Possible mechanism for the incidence of cognitive impairment in people with type 2 diabetes from the standpoint of diabetic vascular disease.
Although an increased risk of cognitive impairment in patients with diabetic retinopathy was found in the reviewed studies, a relationship between severity of diabetic retinopathy and cognitive impairment has not been established3. Also, in Diabetes Care, Crosby-Nwaobi et al.4 recently published findings opposite to those that had previously been reported. They stated that cognitive impairment was not associated with the degree and severity of diabetic retinopathy, and on the contrary, that cognitive decline was greater in patients with no and mild retinopathy as compared with those with advanced retinopathy. In their study, diabetic patients with advanced retinopathy, as compared with those with no and mild retinopathy, had a comparably longer duration of diabetes, higher levels of glycosylated hemoglobin A1c, frequent nephropathy and cardiovascular disease, which are reportedly associated with cognitive impairment. In contrast, education levels were lower in patients with no and mild retinopathy. Naturally enough, the effects of such confounders cannot be disregarded, although the study was adjusted for them. Another issue of their study was that they evaluated patients with no and mild retinopathy together, as the number of patients with no retinopathy was limited, and they also excluded severe retinopathy. It is likely that subtle changes in endothelial cells have already occurred in mild retinopathy and, therefore, differences between no and mild retinopathy should be compared. Also, as it has recently been reported that flow disturbances within the ocular blood vessels of patients with type 2 diabetes were atherogenic changes of coronary arteries, further studies on a relationship between ocular blood vessel flow and cognitive function are desirable.
In contrast, it is generally agreed that chronic kidney disease might be associated with cognitive impairment. However, chronic kidney disease involves albuminuria and estimate glomerular filtration rate (eGFR) decline, and it is still controversial as to which of these markers is more strongly associated with cognitive function. It has been reported that blockers of the renin–angiotensin system decreased albuminuria and protected against cognitive decline as well. In addition, parallel changes in severity and improvement of chronic kidney disease and some cognitive functions have been reported in the recent subanalysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial5. In this regard, we reported that changes in albuminuria, but not eGFR, were strongly correlated with changes in word recall during a 3-year follow up in elderly diabetic patients6. However, in that study, a cross-sectional evaluation of an association between cognitive functions and albuminuria or eGFR produced different results from those of a longitudinal evaluation. Results appear to differ depending on which parameter, eGFR or albuminuria, is used and whether it is a cross-sectional or a longitudinal study. Such disagreement could also be seen in an association between retinopathy and cognitive function. Unfortunately, regarding an association with retinopathy, there have been few longitudinal studies, and to our knowledge there has been no study on an association between changes in cognitive function and retinopathy as a result of therapy. If the findings of Crosby-Nwaobi et al.4 are verified, it is likely that other factors might be present in the relationship with retinopathy, unlike nephropathy. As retinal abnormalities could have a more direct influence in brain damage through disruption of the blood–brain barrier in the retina, there might be some differences in the effect on cognition between retinopathy and nephropathy. There have been a few reports regarding an association between peripheral neuropathy and cognitive impairment. However, interestingly, it has been reported that progression of diabetic nephropathy and retinopathy depended on the final severity of diabetic neuropathy7. Therefore, it is likely that diabetic neuropathy is also associated with cognitive decline. In any case, further prospective studies on these associations will be required.
Recently, many studies have been carried out on the early detection of cognitive impairment, but, unfortunately, they have produced hardly any useful and simple clinical tools. Most clinicians will agree that preventing the incidence or advance of microangiopathy can play an important role in maintaining quality of life and reducing mortality in patients with diabetes mellitus. Also, as microangiopathy is routinely evaluated in the clinical setting, if an association between cognitive impairment and microangiopahy is clarified, and a reduction of microangiopathy reduces the rate of cognitive decline, the monitoring and treatment of microangiopathy could possibly be a tool for preventing cognitive decline in people with diabetes.
Acknowledgments
The authors have no conflicts of interest to declare.
References
- Kawamura T, Umemura T, Hotta N. Cognitive impairment in diabetic patients: can diabetic control prevent cognitive decline? J Diabetes Invest. 2012;3:413–423. doi: 10.1111/j.2040-1124.2012.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura T, Kawamura T, Umegaki H. Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small-vessel disease and cognitive impairment: a 6-year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry. 2011;82:1186–1194. doi: 10.1136/jnnp.2010.217380. [DOI] [PubMed] [Google Scholar]
- Crosby-Nwaobi R, Sivaprasad S, Forbes A. A systematic review of the association of diabetic retinopathy and cognitive impairment in people with Type 2 diabetes. Diabetes Res Clin Pract. 2012;96:101–110. doi: 10.1016/j.diabres.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Crosby-Nwaobi RR, Sivaprasad S, Amiel S. The relationship between diabetic retinopathy and cognitive impairment. Diabetes Care. 2013;36:3177–3186. doi: 10.2337/dc12-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay J, Lovato JF, Murray AM. Albuminuria and cognitive decline in people with diabetes and normal renal function. Clin J Am Soc Nephrol. 2013;8:1907–2013. doi: 10.2215/CJN.11321112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Umemura T, Umegaki H. Effect of renal impairment on cognitive function during a 3-year follow up in elderly patients with type 2 diabetes: association with microinflammation. J Diabetes Invest. 2014;5:597–605. doi: 10.1111/jdi.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta N, Kawamori R, Fukuda M. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med. 2012;29:1529–1533. doi: 10.1111/j.1464-5491.2012.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


