Abstract
The emergence of fluoroquinolone resistance among A. baumannii isolates is now of particular concern. Phenotypic and genotypic characteristics of resistance to ciprofloxacin among 50 Acinetobacter baumannii isolated from burn wound infections of Tehran were evaluated by E-test and broth microdilution in presence and absence of efflux pump inhibitor phenylalanine- arginine β-naphthylamide (PAβN) and PCR-sequencing methods. All isolates were then typed by REP-PCR fingerprinting to find the clonal relationship between resistant isolates.
Our results indicated that resistance to ciprofloxacin among A. baumannii isolated from burn infections in Tehran are high with resistance rate of 100% and ciprofloxacin resistant isolates have a mutation of Serine 83 →Leucine in the quinolone resistance determining region (QRDR) of DNA gyrase subunit A (GyrA). 38% of the isolates showed MIC ranges of 64 to ≥512μg/ml and were considered as highly resistant. We could not detect Par C mutations and plasmid-mediated quinolone resistance A (qnrA) among ciprofloxacin resistant isolates. When we used the efflux pump inhibitor PAbN, MIC of ciprofloxacin was reduced two-to four folds. REP-type A (25/50; 50%), B (20/50; 30%) and C (10/50; 20%) were the most common REP-types among A. baumannii isolates.
It seems that mutation in GyrA is the main mechanism of resistant to ciprofloxacin among A. baumannii isolates from burn infections and presence of efflux pumps is just secondary target for ciprofloxacin resistant among A. baumannii in Iran. Regarding with limitation of REP-types detected in this study, we found good correlation between resistance to ciprofloxacin and REP-types A-C.
Keywords: ciprofloxacin resistance, A. baumannii, gyr A QRDRS
INTRODUCTION
Acinetobacter baumannii is an important nosocomial pathogen causing a wide range of infections in patients in intensive care units (ICUs) and burn wards (1-2). Effective treatment of infections caused by this organism is compromised by a high level of resistance to antimicrobials exhibited by the hospital strains. The emergence of resistance against aminoglycoside, carbapenem, and other beta-lactams in A. baumannii strains has become a major concern in Iran and other parts of the world; therefore, a survey on the effect of suitable antimicrobial agents against these multidrug resistant strains can be beneficial in the therapeutic process of such infectious agents (3-6). Until 1988, the new fluorinated quinolones presented very good activity against Acinetobacter strains and had even a better effect than the expanded-spectrum cephalosporins or aminoglycosides (7). However, resistance to these antibiotics rapidly arose in the clinical isolates especially in Tehran hospitals (4-5). The emergence of fluoroquinolone resistance is now of particular concern, given that relatively few antimicrobial agents are effective against A. baumannii isolated from burns wound infections.
Resistance normally involves chromosomal mutations in the quinolone resistance determining regions (QRDRs) of either one or both of the DNA gyrase or topoisomerase IV (parC) genes that represent the primary and secondary intracellular targets for this class of antibiotics, but a recent study has suggested that a non-specific efflux pump mechanism could also contribute to quinolone resistance in A. baumannii (7-9).
According to the literature review, there are no comprehensive data regarding the mechanisms of resistance against fluoroquinolone antibiotics such as ciprofloxacin among A. baumannii isolates in Iran. So, in this study we examined ciprofloxacin resistance and the mechanisms of resistance including mutation in gyr A, parC and qnr A and contribution of efflux pump among different genotypes of A. baumannii isolated from Tehran hospital of Iran. ❑
MATERIAL AND METHODS
Study population
A total number of 50 A. baumannii isolates were recovered from burns infections during 2010-2012 in Tehran, Iran. The isolates were non repetitive, meaning that each isolate was obtained from a particular patient and each patient was sampled only once. All the isolates were identified as A. baumannii species by routine biochemical methods, API20NE system and by the PCR detection of blaOXA-51-like gene according to previous reports (4-5,10).
Antimicrobial Susceptibility
MIC of ciprofloxacin was determined by both E- test and microbruth dilution the results were explained by CLSI guidelines (11). Test concentrations for antibiotics were 512 µg/ml, 256 µg/ml, 128µg/ml, 64µg/ml, 32µg/ml, 16µg/ml, 8µg/ml, 4µg/ml, 2µg/ml, 1 µg/ml, 0.5µg/ml, 0.25 µg/ml, 0.125 µg/ml. Each well of 96-well microtiter plate contained a total volume of 100µl including antibiotics and Mueller-Hinton medium with the bacterial inoculums. Microplates were stacked four high, covered in plastic wrap to reduce evaporation, and incubated at 35°C for 18 to 24 h. Plates were read visually using an inverted mirror to detect growth at the bottoms of wells. The lowest concentration of antibiotic that did not have visible bacterial growth was defined as the MIC (11).
Effects of efflux pump inhibitors on ciprofloxacin resistance.
Susceptibility to ciprofloxacin in the presence of efflux pump inhibitor was tested as described previously (12). Susceptibility to ciprofloxacin was tested in parallel in the presence and absence of the efflux pump inhibitor PAβN.
Following the addition of ciprofloxacin and the bacterial cell inoculums, 2 µl of the 5-mg/ml stock of either PAβN was added to the microplate wells (total volume, 100 µl). The rest of the procedures were carried out like MIC detection of ciprofloxacin.
PCR amplification and sequencing
The genes gyrA, parC and the plasmid-mediated quinolone resistance (qnrA) from highly ciprofloxacin resistant A. baumannii isolates were amplified by PCR. DNA extraction was done by DNA extraction kit (BIONEER Inc, Republic of Korea). The following parameters were used: an initial template denaturation at 95°C for 1 min; 36 cycles of denaturation at 95°C for 30 s, annealing for gyr A at 52°C for 30 s, for parC at 60°C for 60 s and for qnrA at 52°C for 60 s, an extension step at 72°C for 2 min, and a final extension at 72°C for 10 min. The PCR primers for the amplification and sequencing of the gyrA QRDR were as follows: forward, 5'-AAATCTGCTCGTGTCGTTGG-3'; reverse, 5'-GCCATACCTACAGCAA TACC-3'(3). The PCR primers for the amplification and sequencing of the parC QRDR were as follows: forward, 5'-AAGCCCGTACAGCGCCGTATT-3'; reverse, 5'-AAAGTTATCTTGCCATTCGCT-3'(3). The primers for the amplification of qnrA gene were as follows: forward, 5'- GGGTATGGATATTATTGATAAAG-3'; reverse, 5'-CT AATCCGGCAGCACTATTA-3'(3). For typing of the isolates by Repetitive Extragenic Palindromic-PCR (REP-PCR), the primer pair REP1, 5'- IIGCGCCGICATCAGGC-3' and REP2, 5'-ACGTCTTATCAGGCCTAC-3' were used to amplify putative REP- like elements in the genomic bacterial chromosomes according to previous report (10).
Amplified products were confirmed by agarose gel electrophoresis to estimate the PCR fragment sizes, followed by the sequencing of both strands of the amplified DNAs using forward and reverse primers. Determination of the nucleotide sequence or sequencing was performed by Macrogen Inc., Republic of Korea. Sequences were aligned and amino acids were deduced using the ClustalW included in the Lasergene MegAlign software package v.6.1 (DNA Star, Inc.). ❑
RESULTS
The results of this study in both E-test and broth microdilution showed that 100% of the isolates were resistant to ciprofloxacin with MIC ranges of 16-512 µg/ml (MIC50: 32 µg/ml; MIC90: 64 µg/ml). As shown in Table 1, totally 19 isolates were highly resistant against ciprofloxacin with MIC ranges of 32- ≥512 µg/ml.
Table 1.
Phenotypic and genotypic characterization of 19 highly ciprofloxacin resistant A. baumannii strains
| no | REP-TYPE | MIC CIP µg/ml | MIC (CIP+ PAβN µg/ml) | Gyr A Ser 83→Leu | Par C | qnrA |
|---|---|---|---|---|---|---|
| 1 | A | 512 | 128 | + | - | - |
| 2 | A | 128 | 64 | + | - | - |
| 3 | B | 128 | 64 | + | - | - |
| 4 | A | 128 | 64 | + | - | - |
| 5 | B | 128 | 64 | + | - | - |
| 6 | B | 512 | 128 | + | - | - |
| 7 | A | 128 | 128 | + | - | - |
| 8 | B | 128 | 32 | + | - | - |
| 9 | B | 512 | 64 | + | - | - |
| 10 | A | 512 | 128 | + | - | - |
| 11 | B | 512 | 64 | + | - | - |
| 12 | B | 64 | 16 | + | - | - |
| 13 | A | 32 | 16 | + | - | - |
| 14 | A | 32 | 16 | + | - | - |
| 15 | C | 512 | 128 | + | - | - |
| 16 | C | 512 | 64 | + | - | - |
| 17 | C | 512 | 64 | + | - | - |
| 18 | A | 512 | 64 | + | - | - |
| 19 | A | 512 | 64 | + | - | - |
CIP: ciprofloxacin; PAβN: phenylalanine- arginine β-naphthylamide
None of the isolates gave positive PCR results for qnrA gene. However, amplification of gyrA yielded PCR products of 344 bp. After removing the primers, the deduced amino acid sequences of the amplified 304 bp fragment comprised 101 amino acid residues. Compared with the wild-type A. baumannii gyrA (ATCC 19606, accession no AF100557), several nucleotide substitutions were observed in A. baumannii isolates, among which only one resulted in amino acid substitution; meaning that the gyrA QRDR of multidrug resistant A. baumannii isolates showed a single mutation of Ser- 83→Leu (number corresponding to E. coli strain K-12, accession no U00096). No mutations were found in the parC gene.
As shown in Table 1, MIC of ciprofloxacin in isolates with high resistance to ciprofloxacin in presence of PAβN was reduced two- to four folds. MIC of most isolates reduced at least one fold. The most isolates that shows highest reduced of MIC in presence of PAβN was strains 9 and 11 respectively (data was shown in Table 1).
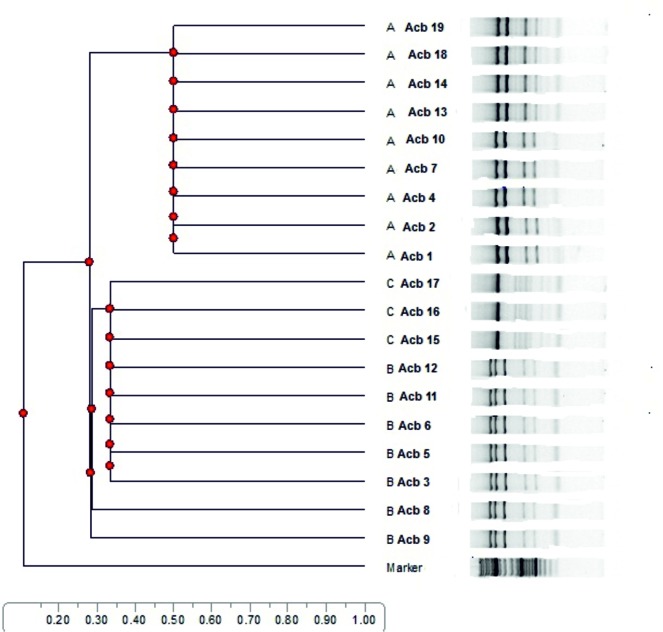
All isolates belong to REP- types A (25/50; 50%), B (15/50; 30%) and C (10/50; 20%) respectively. As shown in Table 1 and Dendrogram 1, distribution of REP- types among 19 highly ciprofloxacin resistant isolates with MIC ≥ 32µg/ml to ciprofloxacin was type A (9/19; 47.4%), type B (7/19; 36.8%) and type C (3/19; 15.8%) respectively. ❑
Dendrogram 1. Distribution of REP types A to C among 19 highly ciprofloxacin resistant A. baumannii isolated from burn wound infections and their clonal relationship.
DISCUSSION
Resistance of A. baumannii to fluoroquinolones has been attributed to changes in the structure of DNA gyrase or topoisomerase IV which are caused by mutations in the gyrA or parC genes, respectively, which lower the affinity of the drug in the enzyme-DNA complex (9-13).
Among our isolates 19 had ciprofloxacin MICs equal or greater than 32 µg/ml, indicating that these isolates were highly resistant to ciprofloxacin. The result of our sequencing showed that all ciprofloxacin resistant isolates had only one mutation in gyrA gene that resulted in Ser-83 to Leu-83 substitution in Gyr A. This result on gyr A mutation was similar to previously reports elsewhere. Most studies have emphasized that both Gyr A and ParC mutations are essential for ciprofloxacin resistant phenotype in A. baumannii isolates (8,12,14). Mutation of Ser 83→Leu in the QRDR of gyrA seems to be the most frequent mutation found in the clinical and laboratory quinolone-resistant isolates of many Gram-negative bacteria including A. baumannii; thus, our finding provides further evidence for such a phenomenon (12,14-16).
A second mechanism of resistance has been described with mutations of chromosomally encoded drug influx and efflux systems that determine intracellular drug accumulation (4,7, 14); these mutations result in either reduced production of outer membrane proteins, which mediate quinolone influx, or stimulation of the cells' efflux system, which leads to active drug expulsion. Multidrug efflux pumps have been recognized as a mechanism of resistance in Gram-negative bacteria (17-19). Efflux pump inhibitors have been shown to reverse multidrug resistance in A. baumannii isolates (20-21).
The result of this study showed that the presence of PAβN resulted in two- to four reduction in the ciprofloxacin MICs in different genotypes of A- to C and non-typable strains and it appear that its presence did not main mechanisms of ciprofloxacin resistant phenotype. Similar to other studies the results were focused on efflux pump inhibitors such PAβN. Nearly in all of these studies PAβN reduced two- to four folds in ciprofloxacin MICs, but it's not consider as primary mechanism of resistance to fluoroquinolones (12,20-21).
Unlike some studies (19,20), in this study we could not detect any qnrA in our clinical isolates of Acinetobacter baumannii. None of the plasmid-mediated Qnr determinants have been identified so far in non-enterobacterial Gram-negative species like Acinetobacter baumannii in Iran. These plasmid-mediated determinants have been identified in many Enterobacteriaceae species throughout the world (22).
Specifically, an amino acid substitution at certain positions in subunits A (GyrA and ParC) of both DNA gyrase and DNA topoisomerase IV, due to point mutations in the QRDRs of the genes encoding these two polypeptides, have been found to contribute to fluoroquinolone resistance. In A. baumannii, the most frequent amino acid substitutions occur at position 83 (Ser-83) of GyrA and at position 80 (Ser-80) of ParC (7-8,14,24). While changes in GyrA are necessary for moderate levels of fluoroquinolone resistance among clinical isolates of A. baumannii, it seems that coexistence of efflux pumps are required in order to achieve high levels of fluoroquinolone resistance (12). ❑
CONCLUSION
The present study showed that resistance rate to ciprofloxacin was increased among A. baumannii strains isolated from burn patients in Tehran, Iran and single mutation in QRDRs codon 83 in gyrA was the main mechanism associated with resistance to ciprofloxacin. Although further studies are needed to elucidate the role of over-expression of efflux pump on ciprofloxacin resistance; however, efflux pump inhibitors reduced MIC of ciprofloxacin two- to four folds and also efflux pump may be considered as the second mechanism of resistance to ciprofloxacin in A. baumannii strains. Finally, it is possible that other mutations at the other locations within gyrA, parC, or other genes contribute to the modulation of the MIC level since gyr A mutation and efflux pumps did not entirely explain resistance.
ACKNOWLEDGMENTS
We gratefully thank all the department laboratories of the Ilam University of Medical Sciences, School of Medicine that participated in this study. This work was supported by Ilam University of Medical Sciences, Vic Chancellor of Research and technology.
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.Oncul O, Oksuz S, Acar A, et al. Nosocomial infection characteristics in a burn intensive care unit: Analysis of an eleven-year active surveillance. Burns. 2014;40:835–41. doi: 10.1016/j.burns.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Lee HG, Jang J, Choi JE, et al. Blood stream infections in patients in the burn intensive care unit. Infect Chemother. 2013;45:194–201. doi: 10.3947/ic.2013.45.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hujer KM, Hujer AM, Hulten EA, et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2006;50:4114–23. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asadollahi K, Alizadeh E, Akbari M, et al. The role of bla(OXA-like carbapenemase) and their insertion sequences (ISS) in the induction of resistance against carbapenem antibiotics among Acinetobacter baumannii isolates in Tehran hospitals. Rouman Arch Microbiol Immunol. 2011;70:153–8. [PubMed] [Google Scholar]
- 5.Asadollahi K, Taherikalani M, Maleki A, et al. Diversity of aminoglycoside modifying enzyme genes among multidrug resistant Acinetobacter baumannii genotypes isolated from nosocomial infections in Tehran hospitals and their association with class 1 integrons. Acta Microbiol Immunol Hung. 2011;58:359–70. doi: 10.1556/AMicr.58.2011.4.11. [DOI] [PubMed] [Google Scholar]
- 6.Gao J, Zhao X, Bao Y, et al. Antibiotic resistance and OXA-type carbapenemases-encoding genes in airborne Acinetobacter baumannii isolated from burn wards. Burns. 2014;40:295–9. doi: 10.1016/j.burns.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Vila J, Ruiz J, Goni P, et al. Mutation in the gyrA gene of quinolone-resistant clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 1995;39:1201–3. doi: 10.1128/aac.39.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vila J, Ruiz J, Goni P, et al. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii. J Antimicrob Chemother. 1997;39:757–62. doi: 10.1093/jac/39.6.757. [DOI] [PubMed] [Google Scholar]
- 9.Spence RP, Towner KJ. Frequencies and mechanisms of resistance to moxifloxacin in nosocomial isolates of Acinetobacter baumannii. J Antimicrob Chemother. 2003;52:687–90. doi: 10.1093/jac/dkg424. [DOI] [PubMed] [Google Scholar]
- 10.Asadollahi P, Akbari M, Soroush S, et al. Antimicrobial resistance patterns and their encoding genes among Acinetobacter baumannii strains isolated from burned patients. Burns. 2012;38:1198–203. doi: 10.1016/j.burns.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Performance Standards for Antimicrobial Susceptibility Testing: Sixteenth Informational Supplement M100-S16. CLSI, Wayne, PA. 2011
- 12.Valentine SC, Contreras D, Tan S, et al. Phenotypic and molecular characterization of Acinetobacter baumannii clinical isolates from nosocomial outbreaks in Los Angeles County, California. J Clin Microbiol. 2008;46:2499–507. doi: 10.1128/JCM.00367-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz J. Mechanisms of resistance to quinolones: target alterations, decreased accumulation and DNA gyrase protection. J Antimicrob Chemother. 2003;51:1109–17. doi: 10.1093/jac/dkg222. [DOI] [PubMed] [Google Scholar]
- 14.Wisplinghoff H, Decker M, Haefs C, et al. Mutations in gyrA and parC associated with resistance to fluoroquinolones in epidemiologically defined clinical strains of Acinetobacter baumannii. J Antimicrob Chemother. 2003;51:177–80. doi: 10.1093/jac/dkf254. [DOI] [PubMed] [Google Scholar]
- 15.Hamouda A, Amyes SG. Novel gyrA and parC point mutations in two strains of Acinetobacter baumannii resistant to ciprofloxacin. J Antimicrob Chemother. 2004;54:695–6. doi: 10.1093/jac/dkh368. [DOI] [PubMed] [Google Scholar]
- 16.Hamouda A, Amyes SG. Development of highly ciprofloxacin-resistant laboratory mutants of Acinetobacter baumannii lacking topoisomerase IV gene mutations. J Antimicrob Chemother. 2006;57:155–6. doi: 10.1093/jac/dki397. [DOI] [PubMed] [Google Scholar]
- 17.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–9. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pages JM, Masi M, Barbe J. Inhibitors of efflux pumps in Gram-negative bacteria. Trends Molecul Med. 2005;11:382–9. doi: 10.1016/j.molmed.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Vila J, Marti S, Sanchez-Cespedes J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2007;59:1210–5. doi: 10.1093/jac/dkl509. [DOI] [PubMed] [Google Scholar]
- 20.Pannek S, Higgins PG, Steinke P, et al. Multidrug efflux inhibition in Acinetobacter baumannii: comparison between 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-beta-naphthylamide. J Antimicrob Chemother. 2006;57:970–4. doi: 10.1093/jac/dkl081. [DOI] [PubMed] [Google Scholar]
- 21.Peleg AY, Potoski BA, Rea R, et al. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother. 2007;59:128–31. doi: 10.1093/jac/dkl441. [DOI] [PubMed] [Google Scholar]
- 22.Touati A, Brasme L, Benallaoua S, et al. First report of qnrB-producing Enterobacter cloacae and qnrA-producing Acinetobacter baumannii recovered from Algerian hospitals. Diagn Microbiol Infect Dis. 2008;60:287–90. doi: 10.1016/j.diagmicrobio.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Wu K, Wang F, Sun J, et al. Class 1 integron gene cassettes in multidrug-resistant Gram-negative bacteria in southern China. Int J Antimicrob agents. 2012;40:264–7. doi: 10.1016/j.ijantimicag.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Lee JK, Lee YS, Park YK, et al. Mutations in the gyrA and parC genes in ciprofloxacin-resistant clinical isolates of Acinetobacter baumannii in Korea. Microbiol immunol. 2005;49:647–53. doi: 10.1111/j.1348-0421.2005.tb03643.x. [DOI] [PubMed] [Google Scholar]