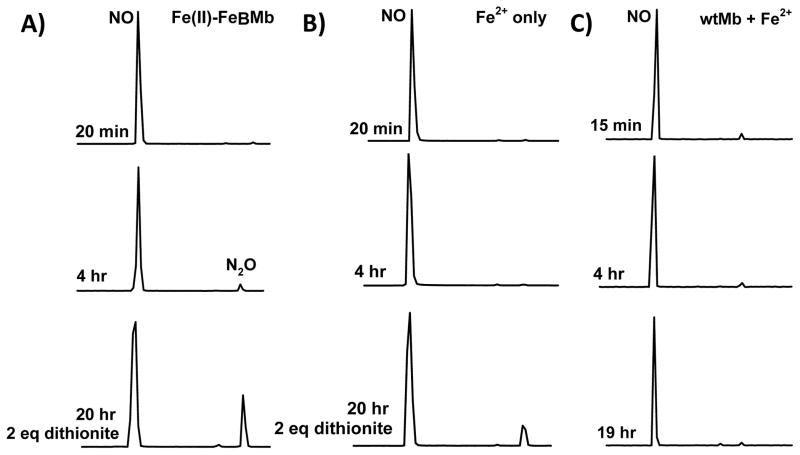
Figure 3.
Product of NO reaction by FeBMb is N2O. Time dependent GC/MS measurements of N2O formation by Fe(II)-FeBMb (A). Control reactions of NO with Fe2+ only (B) and wtMb (C). NO (~17 eq) was reacted with Fe(II)-FeBMb (0.6 mM protein, 1.5 mM or 2.5 eq Fe2+), Fe2+ (1.5 mM, no protein), or with wtMb (1 mM) and Fe2+ (2 mM or 2 eq). At 6 hr, 2 eq dithionite (1.2 mM) was added and allowed to react to simulate a second turnover. NO2 (MW 46) was not detected. GC peaks have been normalized. N2O yield (30%) was determined from a comparison of the ratio of the NO:N2O peaks of the 30 MW:44 MW single ion chromatograms 2 hr after dithionite addition (after which additional N2O formation was not observed), to that of known dithionite concentrations (2 hr after addition). Background N2O formation (i.e., Fe2+ without protein) was subtracted from that of Fe(II)-FeBMb to estimate the yield.