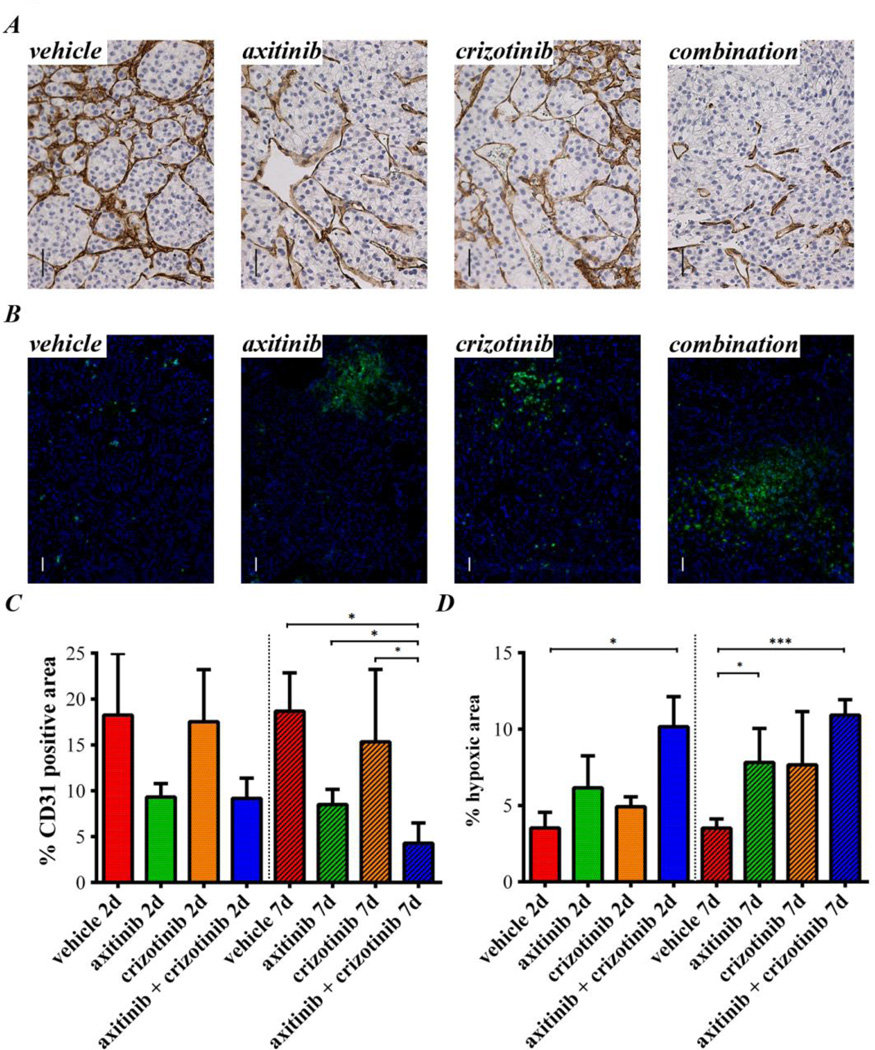
Figure 2. Axitinib and crizotinib short term treatment in the sunitinib sensitive RP-R-01 PDX model.
Subcutaneous RP-R-01 bearing mice (3/group) were treated with vehicle, axitinib (36 mg/kg, 2×/day), crizotinib (25 mg/kg, 1×/day) or combination for either 2 (labeled as 2d) or 7 days (7d). Tumors were then harvested, processed, and tissue sections were stained by immunohistochemistry for (A) CD31, to display endothelial cells and (B) pimonidazole by immunofluorescence (green), to assess intra-tumor hypoxia (DAPI counterstain marks nuclei in blue). Scale bar, 50µm. Blinded quantitative analysis of (C) tumor vascularization and (D) intra-tumor hypoxia, expressed as mean percentage positive stained area ± S.E,. * p <0.05, ***p <0.001, using two tailed t-test analysis (the vertical spotted lines highlights the difference in time among groups).