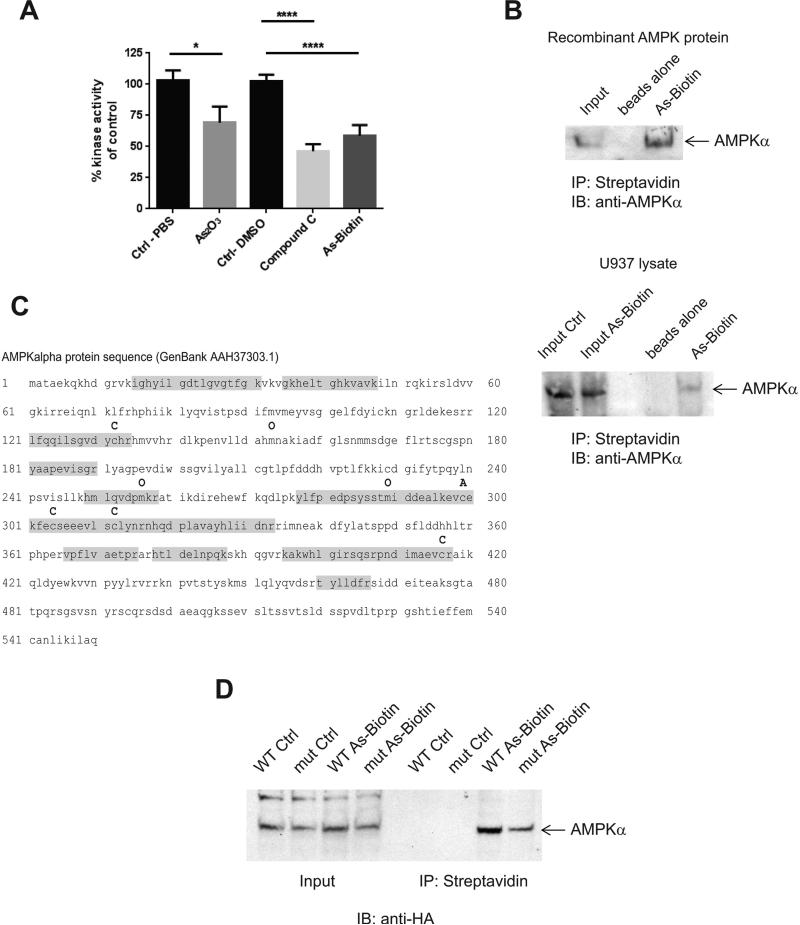
Figure 2.
Arsenic binds directly to AMPK and inhibits its kinase activity. A, An AMPK kinase assay was performed with AMPKα1/β1/γ1 recombinant protein using an ADP-Glo Kinase Assay. Drugs were used at a final concentration of 3 μM. * p <0.05, **** p<0.0001 using an unpaired t-test. B, Recombinant AMPK protein was treated for 30 min at 30° C with As-Biotin (20 μM) to assess in vitro binding of arsenic to AMPK. For in cell binding experiments U937 cells were treated with As-Biotin(20 μM) for 2 hrs and subsequently lysed. Arsenic-biotin complexes were incubated with streptavidin beads overnight and then washed and eluted from the beads with 2 x Laemelli sample buffer. Samples were then subjected to SDS-PAGE, followed by immunoblotting for AMPKα. C, Mass spectroscopy was performed on recombinant AMPK protein and As-Biotin complexes. Shown is the AMPKα protein sequence annotated with the modifications found. C = cysteine unmodified O= oxidation and A= cysteine with As-Biotin. Highlighted in gray is the peptide coverage. D, HA tagged AMPKα WT and AMPKα C299,304A mutant proteins were over-expressed in HEK293 cells. Cells were treated with 20 μM As-Biotin for 2 hrs and subsequently lysed. Arsenic-protein complexes were isolated by incubation with streptavidin beads overnight and then washed and eluted from the beads with 2 x Laemelli sample buffer. Samples were then subjected to SDS-page, followed by western blotting for HA tag.