Abstract
BACKGROUND
β-blockade (BB) has been shown to prevent bone marrow (BM) dysfunction after trauma and hemorrhagic shock (HS). The impact of the sympathetic system and the role of BB on shock-induced distant organ injury is not known. This study will determine if BB has systemic effects and can diminish gut and lung injury after trauma and HS.
METHODS
Male Sprague-Dawley rats were subjected to lung contusion (LC) followed by 45 minute of HS. Animals (n = 6 per group) were then randomized to either receive propranolol (LCHS + BB) immediately after resuscitation or not (LCHS). Gut permeability was evaluated in by diffusion of Mr 4,000 of fluorescein dextran (FD4) from a segment of small bowel into peripheral blood. Villous injury and lung injury were graded histologically by a blinded reader. Plasma-mediated effects of BB were evaluated in vitro by an assessment of BM progenitor growth.
RESULTS
Animals undergoing LCHS had significantly higher plasma levels of FD4 compared with control animals (mean [SEM], 2.8 [0.4] µg/mL vs. 0.8 [0.2] µg/mL). However, animals receiving BB had a significant reduction in plasma FD4 compared with the LCHS group. With the use of BB after LCHS, both ileal and lung injury scores were similar to control. In addition, BM progenitor growth was inhibited by the addition of LCHS plasma, and LCHS + BB plasma showed no inhibition of BM progenitor growth.
CONCLUSION
Propranolol can protect against the detrimental effects of trauma and HS on gut permeability, villous, and lung injury. The effects of BB are likely systemic and appear to be mediated through plasma. BB likely blunts the exaggerated sympathetic response after shock and injury. Propranolol’s reduction of both BM dysfunction and distant organ injury further demonstrates the importance of the sympathetic nervous system and its role in potentiating end organ dysfunction after severe trauma.
Keywords: Propranolol, bone marrow, anemia, trauma
Severe traumatic injury and hemorrhagic shock (HS) are accompanied by increased sympathetic stimulation that leads to the development of a persistent anemic state that may last up to 2 weeks after injury.1,2 This hypercatecholamine state after trauma and HS is associated with increased hematopoietic progenitor cell (HPC) mobilization from the bone marrow (BM) and suppression of BM HPC growth, thus contributing to the anemic state.3,4 After injury and HS, nonselective β-blockade (BB) with propranolol has been shown to not only decrease the mobilization of the HPCs and restore BM HPC growth but also lessen the severity of anemia.4
Multiple-organ failure, which may include BM dysfunction, is a common complication after severe trauma and HS.5 Trauma and HS result in an inflammatory state and an increase in circulating cytokines both systemically and at the site of injury. This inflammatory state has been shown to lead to the development of both gut and lung dysfunction.6 Gut barrier dysfunction has been well described as a component of HS-induced distant organ failure.6–8 Gut barrier dysfunction is manifested as a loss of the villous architecture and mucosal integrity, leading to bacterial translocation, and is associated with the release of proinflammatory gut-derived factors.7,8 Similarly, after hemorrhage and resuscitation, lung dysfunction is manifested by increased neutrophil numbers, histologic changes, and changes in pulmonary function.9 The degree of distant organ injury has been shown to be proportional to the amount of hemorrhage.10–12 Both mesenteric lymph and circulating plasma are potential systemic mediators of toxicity and organ dysfunction after trauma and HS.1,6,13 Previously, circulating plasma from patients with trauma and shocked rats as well as mesenteric lymph have all been shown to suppress BM progenitor cell growth as compared with controls, thus demonstrating that BM dysfunction is systemically mediated.1,13 Mortality in patients with critical illness and failure of four organ systems approaches 100%; therefore, the search for interventions that reduce or prevent organ dysfunction is ongoing.14
Regulation of the catecholamine response with the use of BB has been studied in several models and has been shown to be beneficial in the setting of burns, cardiac disease, and traumatic brain injury.15–17 In addition, we have previously shown that the use of BB can reduce BM dysfunction associated with injury and HS.3,4 However, the impact of the sympathetic system and the role of BB on shock-induced distant organ injury is not known. Therefore, the aim of this study was to determine if BB use has systemic protective effects and can diminish gut and lung injury after trauma and HS.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 300 g to 400 g were housed under barrier-sustained conditions and kept at 25°C with 12-hour light-dark cycles. Animal were provided ad libitum access to water and food (Teklad22/5 Rodent DietW-8640, Harlan Teklad, Madison, WI). The animal facility environment and animals were maintained in accordance with the regulations detailed in the Guide for the Care and Use of Laboratory Animals. The New Jersey Medical School Animal Care and Use Committee approved all animal protocols.
Reagents
Sodium pentobarbital was purchased from Lundbeck, Inc. (Deerfield, IL), and heparin was obtained from Hospira, Inc. (Lakefront, IL). Propranolol hydrochloride (BB), bovine serum albumin, cold MEM-alpha media, and 2-mercaptoethanol were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum, Iscove’s Modified Dulbecco’s Medium, gluta-mine, penicillin/streptomycin, and trypan blue were obtained from Invitrogen (Carlsbad, CA). Methylcellulose was purchased from Stemcell Technologies (Vancouver, Canada). Cytokines, rhEpo, rhIL-3, rhGM-CSF were purchased from R&D (Minneapolis, MN).
Experimental Design
To study the effects of BB on shock-induced distant organ injury, animals were assigned to the following experimental groups (n = 6 animals per group): unmanipulated control (UC), lung contusion followed by HS (LCHS), and LC and HS followed by BB (LCHS + BB). After injury and shock, animals were resuscitated with shed blood. Animals were killed at 3 hours after resuscitation via direct cardiac puncture. To induce BB, animals received an intraperitoneal dose of pro-pranolol at 10 mg/kg after adequate resuscitation from shock (mean arterial pressure [MAP] ≥ 80 mm Hg). Peripheral blood, BM, the right contused lung, and small bowel were collected at the time of the kill.
Combined Lung Contusion and Hemorrhagic Shock Model
Experimental animals were weighed and anesthetized with intraperitoneal injections of sodium pentobarbital (50 mg/kg). The femoral artery and the internal jugular vein were cannulated with polyethylene (PE-50, Becton Dickinson, Sparks, MD) and Silastic (Dow Corning, Midland, MI) tubing, respectively, using aseptic techniques. The femoral artery catheter was used for invasive MAP and heart rate monitoring (BP-2 Digital Blood Pressure Monitor, Columbus Instruments, Columbus, OH). After cannulation of the vessels, the animals were allowed to equilibrate for 10 minutes to 15 minutes. Unilateral LC was inflicted using a blast wave of a percussive nail gun (Craftsman 968514 Stapler, Sears Brands Chicago, IL) applied to a 12-mm metal plate adherent to the right axilla of the rat. This model has been shown to produce aclinically significant LC as demonstrated by roentgenography and histologic examination.18 The animals were then allowed to recover from the LC until their MAP and heart rates return to baseline (10–15 minutes). Subsequently, animals were bled to a MAP of 30 mm Hg to 35 mm Hg for 45 minutes. Temperature was maintained at approximately 37°C with the use of an electric heating pad under the surgical platform. Shed blood was also maintained at approximately 37°C and was reinfused at a rate of 1mL/min after the HS period. Animals assigned to the LCHS + BB group received propranolol after resuscitation with shed blood.
Peripheral Blood
Peripheral blood obtained via direct cardiac puncture was then centrifuged at 10,000 rpm for 10 minutes at 10°C to obtain isolated plasma samples. Plasma samples for fluorescein dextran (FD4) were analyzed immediately. Additional plasma samples from each experimental group (UC, LCHS, and LCHS + BB) were stored at −80°C and used in subsequent analysis described later.
Bone Marrow Progenitor Cell Cultures
To study the effect of circulating plasma on normal BM progenitor cell growth, plasma from each of the experimental groups was evaluated using a standard in vitro BM culture method assessing the growth of viable BM progenitor cells in semisolid media. Normal BM was harvested from all rodent UC femurs by removing the epiphysis and flushing each femur with 5 mL of cold MEM-alpha medium. The BM suspension was then centrifuged at 1,500 rpm (400g) for 15 minutes, the supernatant was discarded, and the remaining pellet was resuspended in 1 mL of Dulbecco’s Modified Eagle’s Medium containing 10% fetal calf serum. Two percent plasma (vol/vol) from UC, LCHS, or LCHS + BB groups was added to the BM suspension. BM mononuclear cells were then plated in duplicate (2 × 106) in Iscove’s media containing 30% fetal calf serum, 2% bovine serum albumin, 1% methylcellulose, rat growth factor, penicillin/streptomycin, 2 × 10−4 mol/L 2-mercaptoethanol, and glutamine. To select for particular erythropoietic progenitor cell lineages, cultures were supplemented with the cytokines 1.3 U/mL rhEpo and 6 U/mL rhIL-3 for erythroid blast–forming unit (BFU-E) and erythroid colony–forming unit (CFU-E) and 3 U/mL rhGM-CSF for granulocyte erythrocyte monocyte megakaryocyte progenitor cells (GEMM). Cultures were incubated at 37°C in 5% CO2, and colonies were counted at 7, 14, and 18 days for CFU-E, BFU-E, and GEMM, respectively, by an observer blinded to the origin of the samples.
Gut and Lung Histologic Examination
The terminal ileum and contused right lungs were harvested and fixed in 10% buffered formalin solution to obtain gut injury scores and lung injury scores for all experimental groups. Processing of samples and histologic grading were performed by a blinded outside consultant. Briefly, after fixation the samples were dehydrated and embedded in paraffin blocks. Four-micrometer thickness sections were cut and stained by hematoxylin and eosin. Gut injury scores were determined based on villous morphology as described previously by Chiu et al.19 Briefly, the degree of injury was graded on a scale of 1 to 5 and ranged from blunting and vacuolization of villous tips to mucosal ulceration (Table 1).19 Lung tissue was assessed using a modified quantitative lung injury score that accounted for inflammatory cells, interstitial edema, pulmonary edema, and alveolar integrity (Table 2).9 The lung injury score ranges from 0 to 11. Slides were evaluated under the standard light microscope, and 30 random fields in each sample were graded in a blinded fashion.
TABLE 1.
Histologic Grading for Gut Injury Scores
| Extent of Intestinal Injury19 | Score |
|---|---|
| Normal mucosa | 0 |
| Subepithelial space and vacuolization at villous tip | 1 |
| Extension of subepithelial space with moderate lifting of epithelial layer from lamina propria | 2 |
| Massive subepithelial lifting and vacuolization from tip to midportion of villi | 3 |
| Epithelial lifting and vacuolization from tip to lower portion of villi | 4 |
| Mucosal ulceration and disintegration of lamina propria | 5 |
TABLE 2.
Histologic Grading for Lung Injury Scores
| Extent of Lung Injury9 | Degree of Injury | Points |
|---|---|---|
| Inflammatory cells/HPF | <5 | 0 |
| 6–10 | 1 | |
| 11–15 | 2 | |
| 16–20 | 3 | |
| >20 | 4 | |
| Interstitial edema | None | 0 |
| Minimal | 1 | |
| Moderate | 2 | |
| Severe | 3 | |
| Pulmonary edema | <5% | 0 |
| 5–25% | 1 | |
| >25% | 2 | |
| Alveolar integrity | Normal | 0 |
| Abnormal moderate | 1 | |
| Abnormal severe | 2 |
HPF, high-power field.
Gut Permeability
To evaluate the effects of shock-induced distant organ injury, gut permeability was assessed in vivo at 3 hours after resuscitation by measuring diffusion of Mr 4,000 FD4 from an isolated segment of small bowel into the peripheral blood. A stock solution of FD4 was prepared at a concentration of 25-mg/mL Dulbecco’s Phosphate-Buffered Saline and kept on ice and protected from light. At 30 minutes before the kill, the animals underwent midline laparotomy and a 10-cm segment of ileum, 3 cm proximal to the ileocecal junction, was isolated by ligation taking care to preserve the distal vascular arcades. Using a 30-gauge needle, 1 mL of stock FD4 solution was then instilled into the isolated loop of bowel, and the puncture site was excluded with ligation to ensure no inadvertent leakage of solution. Thirty minutes after instillation of FD4, the animals are harvested by direct cardiac puncture, and the desired specimens are obtained as described. After separation of plasma via centrifugation, 200-µL aliquot samples were analyzed in a 96-well plate with a microplate fluorescence spectrophotometer (Flx800, Bio-Tek Instruments, Winooski, VT) set to excitation of 485/20, emission 528/20, and sensitivity of 40. Samples were run in duplicate, and values were derived from a standard curve created from serially diluted stock solution.
Statistical Analysis
All data are represented as mean (SEM). The data were subjected to repeated-measures one-way analysis of variance followed by Tukey-Kramer’s multiple comparison post test using GraphPad Prism statistical package (version 4.0, GraphPad, San Diego, CA). A p < 0.05 was considered significant. *p < 0.05 versus UC and **p < 0.05 versus LCHS.
RESULTS
Evaluation of Gut Permeability
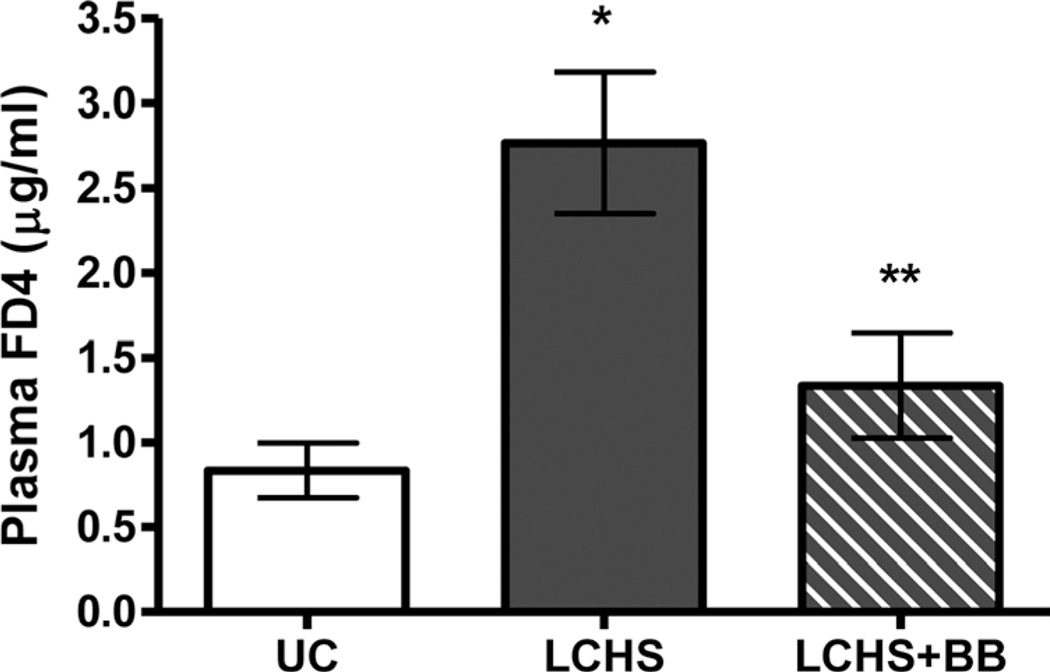
Intestinal barrier integrity was tested 3 hours after LCHS and resuscitation by measuring plasma levels of FD4 to estimate intestinal permeability. LCHS led to increased gut permeability with significantly higher plasma FD4 levels as compared with UC (2.8 [0.4] µg/mL vs. 0.8 [0.2] µg/mL, p < 0.05) (Fig. 1). The addition of BB to LCHS (LCHS + BB) led to a significant reduction in intestinal permeability as measured by the plasma level of FD4 when compared with LCHS alone (1.3 [0.3] µg/mL vs. 2.8 [0.4] µg/mL, p < 0.05) (Fig. 1).
Figure 1.
Effect of propranolol on gut permeability after trauma and shock. n = 6 animals per group. *p < 0.05 versus UC. **p < 0.05 versus LCHS.
Histologic Assessment of Gut and Lung Injury
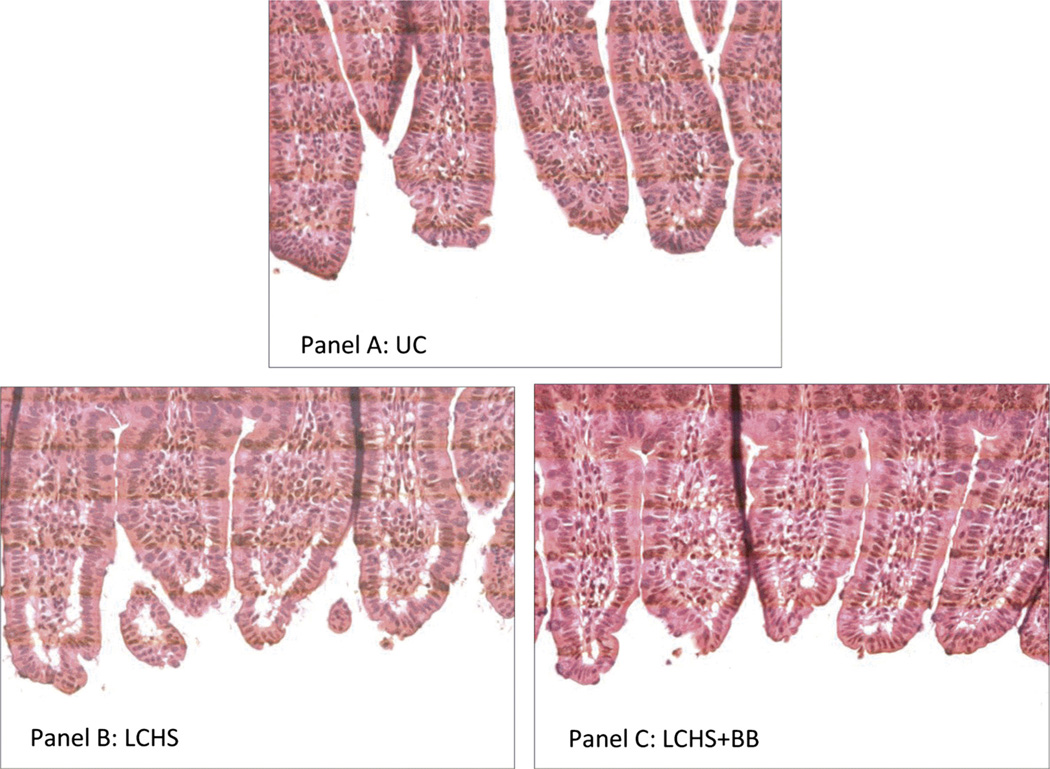
To further assess the effects of BB on the intestinal injury, sections of distal ileum were evaluated in UC, LCHS, and LCHS + BB animals. Figure 2A demonstrates the normal appearance of intestinal villi in the UC animals. The distal small intestine from LCHS animals displayed histologic morphology characterized by epithelial lifting and vacuolization of the villi (Fig. 2B). The gut injury scores in the LCHS animals were elevated compared with UC (2.4 [0.5] vs. 1.4 [0.4]). The protective effects of BB after LCHS are seen with decreased vacuolization of villi and minimal subepithelial changes (Fig. 2C). The gut injury scores in the LCHS + BB animals was not as elevated as those of LCHS-alone animals (1.5 [0.3] vs. 2.4 [0.5]).
Figure 2.
A–C, Effect of propranolol on histologic gut injury after trauma and shock. Sections of distal ileum were stained with hematoxylin and eosin. Images are taken at 400× magnification. A, Representative image from a naive control animal with normal villous architecture. B, Representative image from an animal after LCHS showing increased vacuolization and lifting of epithelium. C, Representative image from animal receiving BB after LCHS and demonstrates decreased vacuolization and minimal subepithelial changes. n = 6 animals per group.
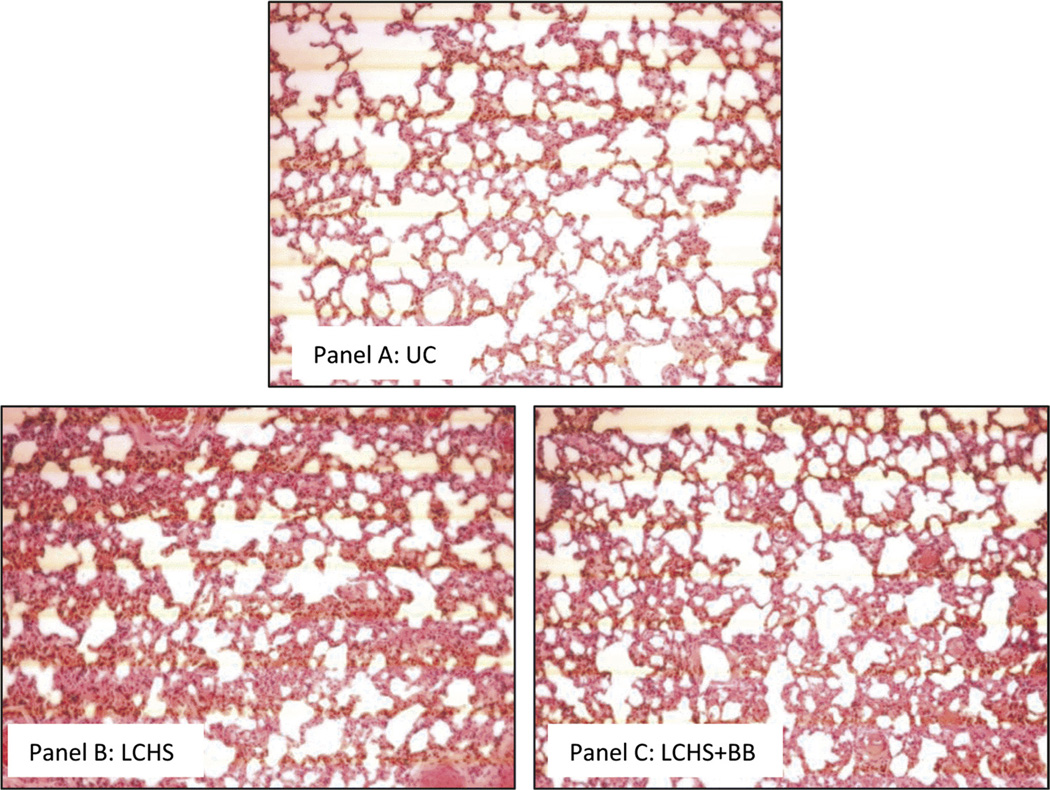
In addition, we examined the effects of BB on injured lung tissue using histologic examination. Figure 3A demonstrates the normal appearance of alveoli in the UC animals. The lung parenchyma after LCHS is characterized by hemorrhage, edema, increased inflammatory cells, and disruption of the alveoli (Fig. 3B) The lung injury scores were significantly elevated in the LCHS group compared with the UC group (8.5 [0.3] vs. 4.0 [0.4], p < 0.05). The protective effects of BB after LCHS are seen in Figure 3C, demonstrating less edema, less inflammatory cells, and less alveolar disruption. In LCHS + BB group, we find that there is a significant reduction in the severity of the lung injury score as compared with that of LCHS alone (4.4 [1.3] vs. 8.5 [0.3], p < 0.05). There is no statistical significant difference in lung injury score between LCHS + BB and UC (4.4 [1.3] vs. 4.0 [0.4]). Looking at all individual parameters of the lung injury score, the degree of inflammation, interstitial edema, and alveolar integrity were all significantly less with the addition of BB (LCHS + BB) as compared with LCHS alone (Table 3).
Figure 3.
A–C, Effect of propranolol on histologic lung injury after trauma and shock. Sections of contused lung were stained with hematoxylin and eosin. Images are taken at 20× magnification. A, Representative image from a naive control animal with normal lung architecture. B, Representative image from an animal after LCHS showing hemorrhage, increased inflammatory cells, edema, and disruption of alveoli. C, Representative image from animal receiving BB after LCHS and demonstrates hemorrhage, with less edema and less inflammatory cells. n = 6 animals per group.
TABLE 3.
Grading of Lung Injury Score 3 Hours After Injury and Shock
| Group (n = 6 per group) | Inflammation (0–4) | Interstitial Edema (0–3) | Intra-alveolar Edema (0–2) | Alveolar Integrity (0–2) | Sum Score |
|---|---|---|---|---|---|
| UC | 2.0 (0.3) | 0.6 (0.3) | 1.0 (0.2) | 0.4 (0.0) | 4.0 (0.3) |
| LCHS | 4.0 (0.0)* | 1.0 (0.0) | 1.7 (0.2) | 1.8 (0.2) | 8.5 (0.3)* |
| LCHS + BB | 2.2 (0.6)† | 0.2 (0.2)† | 1.2 (0.2) | 0.8 (0.4)† | 4.4 (1.3)† |
p < 0.05 versus UC.
p < 0.05 versus LCHS.
LCHS + BB, lung contusion and HS followed by BB after resuscitation; LCHS, lung contusion followed by HS.
Plasma-Mediated Systemic Effect of BB on BM Progenitor Cells
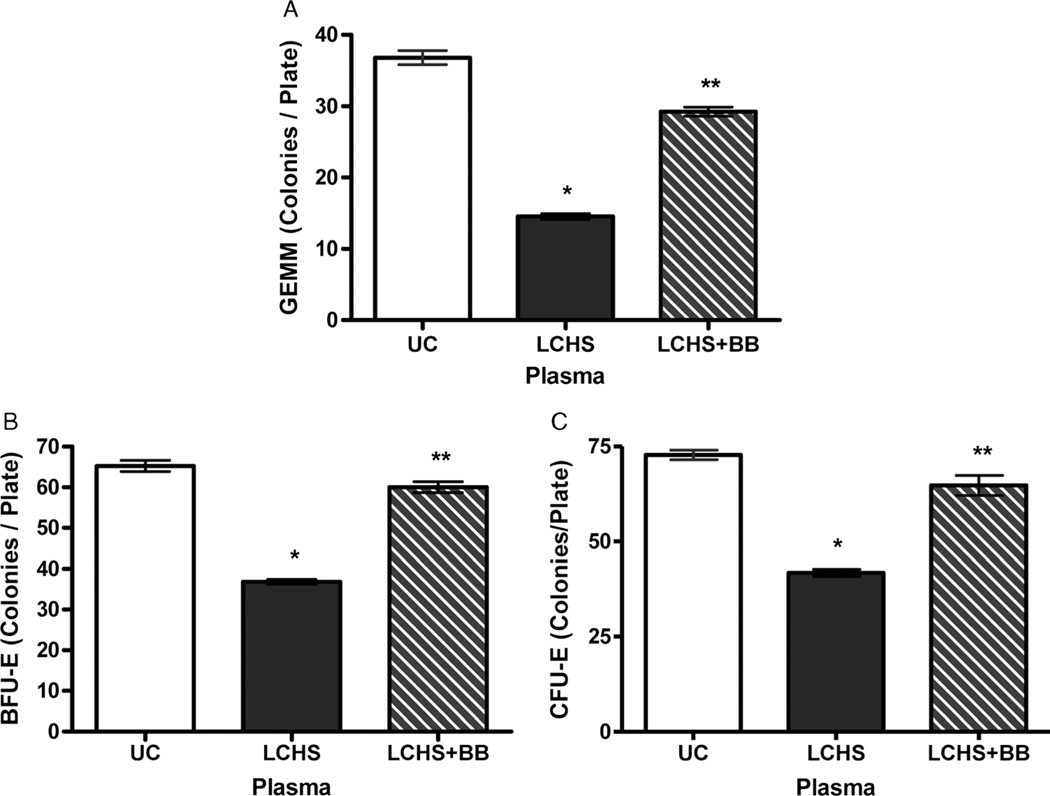
To further evaluate the systemic effects of BB after LCHS, plasma from experimental groups was cultured with normal rodent BM in vitro. Plasma from rats subjected to LCHS alone significantly suppressed BM progenitors, CFU-GEMM, BFU-E, and CFU-E growth as compared with that of UC (15 [1], 37 [1], 42 [1] vs. 37 [1], 65 [1], 73 [1], p < 0.05) (Fig. 4A–C). Plasma from LCHS + BB animals prevented the suppression of all BM progenitors as compared with that of LCHS animals (Fig. 4A–C). The plasma from BB-treated animals nearly restored CFU-GEMM, BFU-E, and CFU-E growth to that of UC animals (29 [1], 60 [1], 65 [3] vs. 37 [1], 65 [1], 73 [1]).
Figure 4.
A–C, Systemic effects of propranolol on BM HPC growth. n = 6 per group. *p < 0.05 versus UC. **p < 0.05 versus LCHS.
CONCLUSION
Recent evidence shows that the use of propranolol can protect the BM and reduce HPC mobilization to the peripheral blood after tissue injury and HS.4 The protective effect of propranolol on the BM appears to be mediated via the β-2 and β-3 adrenergic receptors and may be linked to a reduction in norepinephrine (NE) after severe injury.20,21 Because the hyperadrenergic state after severe traumatic injury is a systemic process, we hypothesized that the beneficial effects of propranolol would not be limited to the BM. This study demonstrates that the use of propranolol after tissue injury and HS attenuates gut and lung injury, gut permeability, and BM HPC growth suppression via a systemic effect.
The exact mechanism of trauma plasma’s BB protection of BM HPC growth is unknown. Fonseca et al.22 established that increasing levels of NE in vitro led to a dose-dependent reduction in erythroid HPC growth. In addition, propranolol has been shown to decrease BM HPC mobilization from BM to peripheral blood after severe injury.4 Livingston et al.1 has also shown that the plasma from patients with severe injury and trauma is inhibitory to BM HPC growth. In this current model of tissue injury followed by HS, the plasma caused a 50% reduction in the growth of all BM HPCs, including GEMM, BFU-E, and CFU-E. The plasma of a patient with trauma inhibited normal BM BFU-E and granulocyte-monocyte growth 40% to 60% for up to 2 weeks after injury.23 These findings suggested that the beneficial effects of propranolol after trauma are potentially systemic. This study demonstrates that the use of propranolol after lung injury and HS protect BM HPC growth and prevent BM suppression associated with injury and shock. Propranolol successfully reduced the systemic toxicity of circulating plasma after lung injury and HS.
The next question to be investigated was whether the plasma protective effects of propranolol systemically reduced organ dysfunction. A link between gut injury, gut-derived factors in mesenteric lymph, lung injury, and BM dysfunction after shock has previously been described by Anjaria et al.13 Studies have demonstrated that the integrity of gut barrier function is compromised by a variety of insults including burns, sepsis, traumatic brain injury, and HS.24,25 For many years, it has been postulated that the integrity of gut barrier function is a key in the development of sepsis after injury and multiple-organ dysfunction.26 This study demonstrates decreased gut permeability correlated with less severe intestinal mucosal injury in the terminal ileum 3 hours after LCHS in animals treated with propranolol after resuscitation. The differences in gut injury scores after treatment with propranolol did not reach statistical significance compared with the LCHS alone. The lack of statistical significance is likely because of the semiquantitative nature of the analysis, small sample size, and a short experimental period of 3 hours. Despite the lack of statistical significance, the gut injury scores with the use of propranolol after LCHS were similar to that of control animals, suggesting that there is a trend toward histologic gut protection with propranolol use. There were minimal histologic changes seen in the control animals (gut injury score, 1.4 [0.4]), which are likely caused by anesthesia and surgical effects related to sampling.
This is the first study to demonstrate a protective effect of gut intestinal permeability and gut injury with the use of propranolol in a model of tissue injury and HS. Several studies targeting additional treatment modalities have demonstrated improvement in gut barrier function. In a burn model, gut barrier injury was reduced by the use of intraperitoneal pentoxifylline.24 Given before traumatic brain injury, intraperitoneal ghrelin use was shown to significantly decrease gut permeability and preserve histologic intestinal architecture.27 Recombinant erythropoietin given intravenously after HS has also been shown to decrease gut mucosal injury, bacterial translocation, and gut intestinal permeability.28 All of these studies demonstrate that the use of systemic agents can reduce intestinal injury after burns, trauma, and hemorrhage.
Likewise, histologic evaluation of the injured lung demonstrates improvement in lung injury scores with the use of propranolol after injury and HS. The improvement in lung injury scores seen may be caused by the systemic protection of propranolol use, or it may be associated with propranolol’s protection of the gut. Primary prevention of gut barrier failure has been demonstrated to prevent pulmonary injury.6 HS producing gut barrier failure may lead to pulmonary injury as a result of reperfusion of the gut. Therefore, agents targeted at decreasing reperfusion injury have been shown to improve acute lung injury, including l-arginine and inhibitors of complement.29,30
The question of what systemic factors potentiate organ dysfunction after severe traumatic injury remains unknown. These data suggest that one candidate is NE. The sympathetic system is known for its mediation of the sympathoadrenal or the “fight or flight” response, which leads to the release of epinephrine and NE during times of stress. Both NE and epinephrine are dramatically increased after trauma and HS and can modulate inflammatory processes including cytokine release.2,31 The proinflammatory effects of epinephrine and NE are mediated through their interaction on both α-adrenergic and β-adrenergic receptors.32 Both α-adrenergic and β-adrenergic blockades have been found to modulate proinflammatory cytokine release and activation of nuclear factor κB in lung cell populations via different mechanisms after hemorrhage and endotoxemia.31,33 In addition, catecholamines increase the generation of reactive oxygen intermediates and contribute to altered vascular perfusion and ischemia/reperfusion injury.34 Thus, a systemic reduction in a severe hyperadrenergic state and NE may help to alleviate posttraumatic organ dysfunction.
As opposed to the sympathetic system, the parasympathetic system acts to maintain homeostasis. Similarly then, rather than reducing sympathetic proinflammatory stimulation, the use of parasympathetic stimulation has produced anti-inflammatory effects,35 leading to reduced organ dysfunction. In a burn model, vagal nerve stimulation before and after injury demonstrated protective effects on the gut mucosal barrier.35 The therapeutic benefit of vagal nerve stimulation in burn injury was limited by a 90-minute window from the time of injury.35 Similarly, stimulation of the vagus nerve after injury has been shown to have protective effects on acute lung injury and inflammatory markers.36
It appears that when the balance of these two autonomic systems is disrupted, as seen with prolonged catecholamine surge after trauma and HS, it is detrimental. It is the magnitude of the injury and the severity of the injury and HS that directly correlates with gut barrier failure and organ dysfunction.10–12 In addition, the adrenal medulla and the nervous system are not the sole producers, storers, and releasers of catecholamines. There is an increasing body of evidence suggesting that lymphocytes and phagocytes synthesize and release neuropeptides, neurotransmitters, and hormones.37 Furthermore, these cells express both adrenergic and cholinergic receptors making them mediators of neuroendocrine-immune system that is a key component of the stress response after severe traumatic injury that have yet to be fully understood.
In summary, this original basic science animal study provides statistically significant level I evidence that LC and HS are associated with BM dysfunction as well as lung and gut injury, and the suppressive effects of tissue injury and HS in the gut and lung were alleviated with the use of propranolol after resuscitation. These observations, combined with the observation that plasma from the propranolol-treated animals prevented suppression of BM HPC growth, strongly suggest that a systemic reduction in catecholamines helps to alleviate organ dysfunction after severe injury. This adds to the evidence that therapeutic strategies that reduce the exaggerated sympathetic stimulation after severe injury and shock are beneficial and reduce organ dysfunction. Further studies are needed to determine the mechanisms involved in propranolol’s prevention of organ dysfunction to better target other future potentially successful therapeutic strategies.
Acknowledgments
DISCLOSURE
This research was supported by the National Institutes of Health grant K08 NIH GM078304 and the Clowes American College of Surgeons/American Association for the Surgery of Trauma Award.
EDITORIAL CRITIQUE
These results represent further investigation from this laboratory into the systemic protective effects of β-blockade following traumatic injury. The authors demonstrate, in an in vivo model of lung contusion and hemorrhage, that gut permeability is increased and lung injury scores are significantly elevated after inducing injury. Post-resuscitation treatment with the β-blocker propranolol resulted in decreased intestinal permeability as well as a reduction in the severity of lung injury scores. In addition, the inhibitory effect of lung contusion and hemorrhagic shock on bone marrow progenitor growth was alleviated by treatment with propranolol. The authors conclude that propranolol administered after traumatic injury reduces organ dysfunction.
The “stress response” following traumatic injury has been of significant interest to researchers. Although the initial sympathetic stimulation produced may confer a survival advantage, there are clear disadvantages to the induction of a persistent inflammatory state. As these investigators have demonstrated, the hyperadrenergic response following trauma causes bone marrow suppression and leads to the development of gut and lung dysfunction. One unanswered question is the mechanism by which propranolol reduces organ dysfunction and the authors acknowledge that this warrants further investigation. However, the most compelling question that emerges is how to translate these results from bench to bedside. If the systemic effects of propranolol in alleviating organ dysfunction can be demonstrated in humans as well, this will profoundly impact the management of critically ill patients. It is important to note that in 2005, Lindenauer and colleagues published the results of a retrospective study on the use of β-blockade in the New England Journal of Medicine. They concluded that the use of β-blockade in low risk patients is potentially harmful. Since many trauma patients fall into this low risk category, this may be something to consider as the authors apply their results clinically.
Nicole Fox, MD, MPH
Department of Surgery
Cooper University Hospital
Haddonfield, New Jersey, USA
Footnotes
This study was presented at the 25th Annual Meeting of the Eastern Association for the Surgery of Trauma, January 10Y14, 2012, in Lake Buena Vista, Florida.
AUTHORSHIP
G.M.B. and A.M.M. performed the literature search. A.M.M. and Z.C.S. designed the study. G.M.B., K.M.C., and W.D.A. collected the data. G.M.B., K.M.C., W.D.A., Z.C.S., D.H.L., and A.M.M. interpreted and analyzed the data. G.M.B., Z.C.S., D.H.L., and A.M.M. wrote the manuscript. G.M.B. prepared the figures.
REFERENCES
- 1.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, Rameshwar P. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–753. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, Livingston DH. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect (Larchmt) 2004;5:385–393. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 3.Elhassan IO, Hannoush EJ, Sifri ZC, Jones E, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Beta blockade prevents hematopoietic progenitor cell suppression following hemorrhagic shock. Surg Infect (Larchmt) 2010;11:214. doi: 10.1089/sur.2010.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohr AM, Elhassan IO, Hannoush EJ, Sifri ZC, Offin MD, Alzate WD, Rameshwar P, Livingston DH. Does beta blockade post injury prevent bone marrow suppression? J Trauma. 2011;70:1043–1050. doi: 10.1097/TA.0b013e3182169326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heckbert SR, Vedder NB, Hoffman W, Winn RK, Hudson LD, Jurkovich GJ, Copass MK, Harlan JM, Rice CL, Maier RV. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998;45:545–552. doi: 10.1097/00005373-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grotz M, Deitch E, Ding J, Xu DZ, Lu Q, Deitch EA. Intestinal cytokine response after gut ischemia: role of gut barrier failure. Ann Surg. 1999;229:478–486. doi: 10.1097/00000658-199904000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deitch EA. Multiple organ failure: pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claridge JA, Enelow RI, Young JS. Hemorrhage and resuscitation induce delayed inflammation and pulmonary dysfunction in mice. J Surg Res. 2000;92:206–213. doi: 10.1006/jsre.2000.5899. [DOI] [PubMed] [Google Scholar]
- 10.Moomey CB, Jr, Melton SM, Croce MA, Fabian TC, Proctor KG. Prognostic value of blood lactate, base deficit and oxygen-derived variables in an LD50 model of penetrating trauma. Crit Care Med. 1999;27:154–161. doi: 10.1097/00003246-199901000-00044. [DOI] [PubMed] [Google Scholar]
- 11.Martel MJ, MacKinnon KJ, Arsenault MY, Bartellas E, Klein MC, Lane CA, Sprague AE, Wilson AK. Clinical Practice Obstetrics Committee and Executive and Council, Society of Obstetricians and Gynaecologists of Canada. Hemorrhagic shock. J Obstet Gynaecol Can. 2002;24:504–520. [PubMed] [Google Scholar]
- 12.Bulger EM, Cuschieri J, Warner K, Maier RV. Hypertonic resuscitation modulates the inflammatory response in patients with traumatic hemorrhagic shock. Ann Surg. 2007;245:635–641. doi: 10.1097/01.sla.0000251367.44890.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anjaria DJ, Rameshwar P, Deitch EA, Xu D, Adams CA, Forsythe RM, Sambol JT, Hauser CJ, Livingston DH. Hematopoietic failure after hemorrhagic shock is mediated partially through mesenteric lymph. Crit Care Med. 2001;29:1780–1785. doi: 10.1097/00003246-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Durham RM, Moran JJ, Mazuski JE, Shapiro MJ, Baue AE, Flint LM. Multiple organ failure in trauma patients. J Trauma. 2003;55:608–616. doi: 10.1097/01.TA.0000092378.10660.D1. [DOI] [PubMed] [Google Scholar]
- 15.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 16.Arbabi S, Ahrns KS, Wahl WL, Hemmila MR, Wang SC, Brandt MM, Taheri PA. Beta-blockade use is associated with improved outcomes in adult burn patients. J Trauma. 2004;56:265–271. doi: 10.1097/01.TA.0000109859.91202.C8. [DOI] [PubMed] [Google Scholar]
- 17.Inaba K, Teixeira P, David JS, Chan LS, Salim A, Brown C, Browder T, Beale E, Rhee P, Demetriades D. Beta-blockers in isolated blunt head injury. J Am Coll Surg. 2008;206:432–438. doi: 10.1016/j.jamcollsurg.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Badami CD, Livingston DH, Sifri ZC, Caputo FJ, Bonilla L, Mohr AM, Deitch EA. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma. 2007;63:596–602. doi: 10.1097/TA.0b013e318142d231. [DOI] [PubMed] [Google Scholar]
- 19.Chiu C, McArdle A, Brown R, Scott H, Gurd F. Intestinal mucosal lesions in low-flow states. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 20.Beiermeister KA, Keck BM, Sifri ZC, Hannoush EJ, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Hematopoietic progenitor cell mobilization is mediated through beta-2 and beta-3 adrenergic receptors following injury. J Trauma. 2010;69:338–343. doi: 10.1097/TA.0b013e3181e5d35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penn A, Mohr AM, Shah SG, Sifri ZC, Kaiser VL, Rameshwar P, Livingston DH. Dose response relationship between norepinephrine and erythropoiesis: evidence for a critical threshold. J Surg Res. 2010;163:e85–e90. doi: 10.1016/j.jss.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fonseca RB, Mohr AM, Wang L, Sifri ZC, Rameshwar P, Livingston DH. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59:884–890. doi: 10.1097/01.ta.0000187653.64300.f5. [DOI] [PubMed] [Google Scholar]
- 23.Wu JC, Livingston DH, Hauser CJ, Deitch EA, Rameshwar P. Trauma inhibits erythroid burst-forming unit and granulocyte-monocyte colony-forming unit growth through the production of TGF-beta1 by bone marrow stroma. Ann Surg. 2001;234:224–232. doi: 10.1097/00000658-200108000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costantini TW, Loomis WH, Putnam JG, Drusinsky D, Deree J, Choi S, Wolf P, Baird A, Eliceiri B, Bansal V, Coimbra R. Burn-induced gut barrier injury is attenuated by phosphodiesterase inhibition: effects on tight junction structural proteins. Shock. 2009;31:416–422. doi: 10.1097/SHK.0b013e3181863080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bansal V, Costantini T, Ryu SY, Peterson C, Loomis W, Putnam J, Elicieri B, Baird A, Coimbra R. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J Trauma. 2010;68:1059–1064. doi: 10.1097/TA.0b013e3181d87373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deitch EA. Role of gut lymphatic system in multiple organ failure. Curr Opin Crit Care. 2001;7:92–98. doi: 10.1097/00075198-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Bansal V, Rye SY, Blow C, Costantini T, Loomis W, Eliceiri B, Baird A, Wolf P, Coimbra R. The hormone ghrelin prevents traumatic brain injury induced intestinal dysfunction. J Neurotrauma. 2010;27:2255–2260. doi: 10.1089/neu.2010.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kao RL, Xenocostas A, Driman DK, Rui T, Huang W, Jiao X, Martin CM. The effect of erythropoietin on microcirculation perfusion and tissue bioenergetics of the small intestine in a hemorrhagic shock and resuscitation rat model. J Trauma. 2010;68:1342–1348. doi: 10.1097/TA.0b013e3181d27dbe. [DOI] [PubMed] [Google Scholar]
- 29.Fu TL, Zhang WT, Zhang L, Wang F, Gao Y, Xu M. l-arginine administration ameliorates serum and pulmonary cytokine response after gut ischemia-reperfusion in immature rats. World J Gastroenterol. 2005;11:1070–1072. doi: 10.3748/wjg.v11.i7.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yazdi CA, Williams B, Oakes S, Moore FD. Attenuation of the effects of rat hemorrhagic shock with a reperfusion injury-inhibiting agent specific to mice. Shock. 2009:295–301. doi: 10.1097/SHK.0b013e3181995e0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arcaroli J, Yang KY, Yum HK, Kupfner J, Pitts TM, Park JS, Strassheim D, Abraham E. Effects of catecholamines on kinase activation in lung neutrophils after hemorrhage or endotoxemia. J Leukoc Biol. 2002;72:571–579. [PubMed] [Google Scholar]
- 32.Le Tulzo Y, Shenkar R, Kaneko D, Moine P, Fantuzzi G, Dinarello CA, Abraham E. Hemorrhage increases cytokine expression in lung mononuclear cells in mice: involvement of catecholamines in nuclear factor-kappaB regulation and cytokine expression. J Clin Invest. 1997;99:1516–1524. doi: 10.1172/JCI119314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham E, Arcaroli J, Shenkar R. Activation of extracellular signal-regulated kinases, NF-kappa B, cyclic adenosine 5′-monophosphate response element-binding protein in lung neutrophils occurs by differing mechanisms after hemorrhage or endotoxemia. J Immunol. 2001;166:522–530. doi: 10.4049/jimmunol.166.1.522. [DOI] [PubMed] [Google Scholar]
- 34.Allen DR, Wallis GL, McCay PB. Catechol adrenergic agents enhance hydroxyl radical generation in xanthine oxidase systems containing ferritin: implications for ischemia/reperfusion. Arch Biochem Biophys. 1994;315:235–243. doi: 10.1006/abbi.1994.1495. [DOI] [PubMed] [Google Scholar]
- 35.Krzyzaniak M, Peterson C, Loomis W, Hageny AM, Wolf P, Reys L, Putnam J, Eliceiri B, Baird A, Bansal V, Coimbra R. Postinjury vagal nerve stimulation protects against intestinal epithelial barrier breakdown. J Trauma. 2011;70:1168–1175. doi: 10.1097/TA.0b013e318216f754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song XM, Li JG, Wang YL, Liang H, Huang Y, Yuan X, Zhou Q, Zhang ZZ. Effect of vagus nerve stimulation on thermal injury in rats. Burns. 2010;36:75–81. doi: 10.1016/j.burns.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA. Catecholaminescrafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening Pandora’s box? Mol Med. 2008;14:195–204. doi: 10.2119/2007-00105.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]