Figure 1.
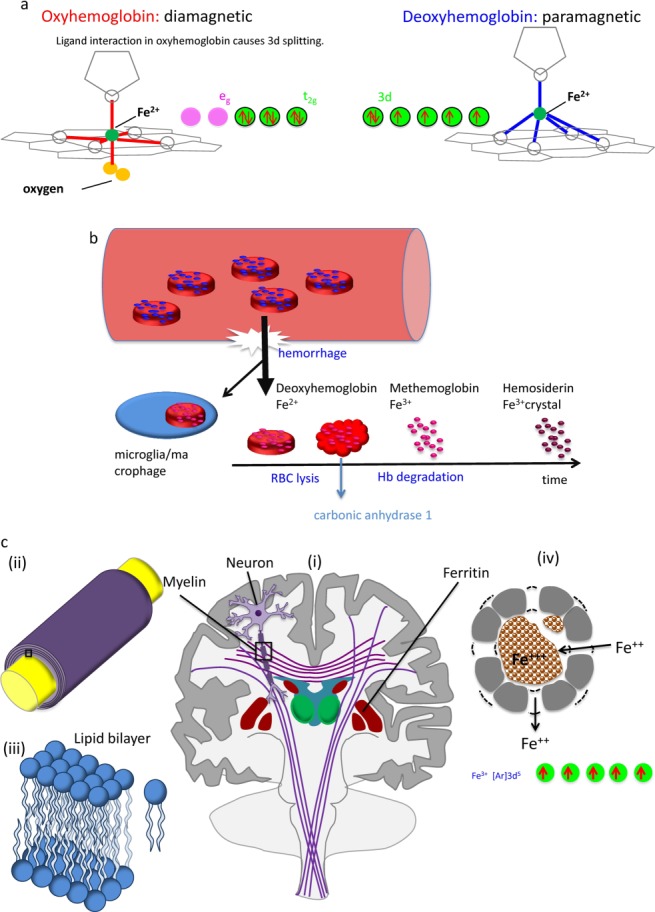
Biomedical magnetic materials. (a) Diamagnetic hemoglobin and paramagnetic deoxyhemoglobin. During metabolic consumption of oxygen in the brain, heart, and kidney, weakly diamagnetic oxyhemoglobin releases O2 and becomes strongly paramagnetic deoxyhemoglobin. Whereas the 3d electron orbits of Fe2+ in deoxyhemoglobin may be approximated as an isolated iron atom with four unpaired electrons (right), the intramolecular interaction between the porphyrin ring and Fe2+ in oxyhemoglobin (ligand interaction) splits the Fe atom’s 3d-orbit into two levels, eg and t2g, with all six electrons paired in the three t2g orbits. (b) Blood degradation in hemorrhage. Following the onset of a hemorrhage, a small fraction of red blood cells (RBCs) may be endocytosed by microglia/macrophages. The majority of RBCs undergo cell lysis and hemoglobin (Hb) degradation from deoxyhemoglobin into methemoglobin (Fe3+) and hemosiderin (possible magnetic domain). Modeled after: Lancet Neurol 2012;11:720–731. (c) Susceptibility sources in the human brain. Major susceptibility sources in (i) the brain include myelin and ferritin. The white matter tracts in the brain consist of myelinated nerve fibers. (ii) Zoomed view of the box in (i) showing axon (yellow) and myelin sheath (purple). Myelin consists of several layers of lipid bilayer. (iii) Zoomed view of the box in (ii) showing a lipid bilayer and an individual lipid. (iv) Ferritin in a cross-section. Ferritin consists of a peptide spherical shell 2-nm thick with a 8-nm diameter cavity. Fe2+ enters through a four-fold symmetric channel, is stored as Fe3+ oxide mineral, and is released as Fe2+ through a three-fold symmetric channel. There are five unpaired 3d electrons in Fe3+, generating strong paramagnetism.
