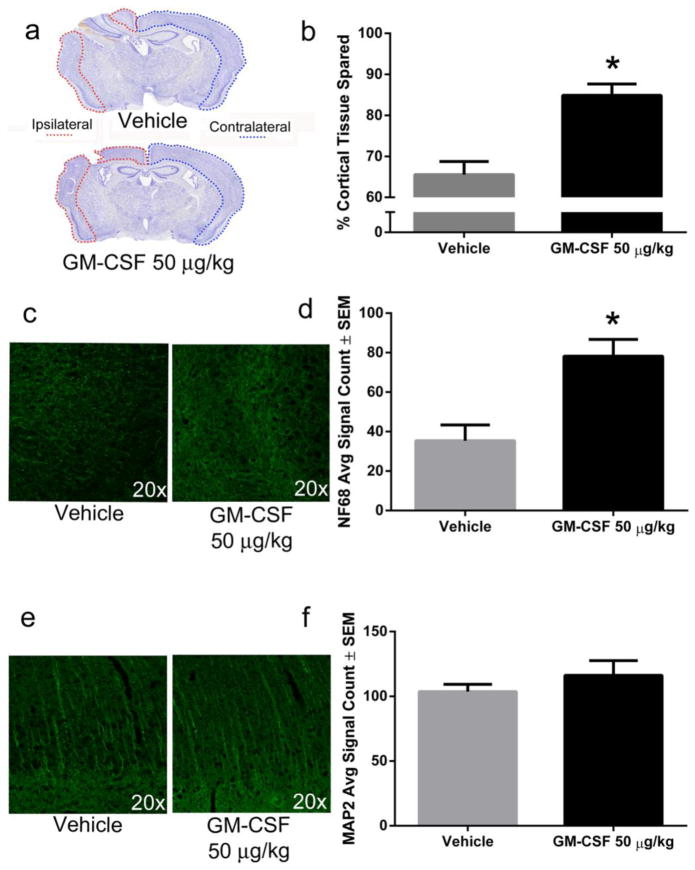
Fig. 1. GM-CSF administration is neuroprotective seven days following CCI.
a) Representative photomicrographs of coronal brain sections stained with cresyl violet show a large lesion in the ipsilateral hemisphere that is reduced by GM-CSF administration. b) Lesion areas were quantified using Image J and demonstrated approximately 19% (p < 0.001) more cortical tissue (relative to the contralateral hemisphere) for GM-CSF treated animals (n = 7) compared to vehicle-treated animals (n = 6). c) Representative photomicrographs of NF68 immunoreactivity in the lesion area of vehicle and GM-CSF treated animals. d) GM-CSF treatment significantly increased NF68 staining by approximately 100% in the lesion area compared to vehicle treatment (p < 0.01). e) Representative photomicrographs of MAP2 immunoreactivity in the lesion area of vehicle and GM-CSF treated animals. f) There was no significant difference in MAP2 staining between vehicle and GM-CSF treated animals in the region of cortical damage.