Abstract
The peripheral immune response contributes to neurodegeneration after stroke yet little is known about how this process differs between males and females. The current study demonstrates that splenectomy prior to experimental stroke eliminates sex differences in infarct volume and activated brain monocytes/microglia. In the periphery of both sexes, activated T cells correlate directly with stroke outcome while monocytes are reduced by splenectomy only in males. This study provides new information about the sex specific mechanisms of the peripheral immune response in neurodegeneration after stroke and demonstrates the need for representation of both sexes in basic and clinical stroke research.
Keywords: Experimental stroke, Sex difference, Immune response, Neuroinflammation, Infarct volume, Splenectomy
1. Introduction
Stroke is one of the leading causes of death and disability in the United States. The role that the immune response plays in neurodegeneration following stroke has been well documented. Leukocyte activation in the spleen after stroke results in a release of immune cells into the blood, infiltration of leukocytes and activation of microglial cells in the brain, leading to an increase in infarct volume (Offner et al., 2006a, Offner et al., 2006b, Seifert et al., 2012a, Seifert et al., 2012b). Long-term systemic immunosuppression is an additional consequence of massive stroke-induced splenocyte activation.
It is now increasingly clear that males and females respond to stroke differently. Females have a lower incidence of stroke and are relatively protected from immediate ischemia compared to males (Sudlow and Warlow, 1997, Alkayed et al., 1998, Murphy et al., 2004). Females also exhibit less stroke-induced splenic damage, which is hypothesized to correlate with their reduced infarct volume (Banerjee et al., 2013). However, females suffer higher rates of disability and handicap over time compared to males (Di Carlo et al., 2003). The underlying molecular and inflammatory mechanisms that lead to stroke-induced sex discrepancy have not been extensively studied. Female mice exhibit a decrease in infiltrating and activated monocytes/microglia in the ischemic brain after MCAO compared to males (Banerjee et al., 2013). In conjunction with splenic damage, males have more activated T cells in the spleen following MCAO than females (Banerjee et al., 2013). Additional pre-clinical data in female animals is needed in order to develop new stroke therapies that will convey clinical success in both sexes.
Although differences in the spleen have been observed between post-MCAO male and female mice, the effect of the spleen on infarct volume and stroke progression is relatively unknown in female animals. Splenectomy in male rodents prior to experimental stroke reduces infarct volume and neurodegeneration by decreasing T cells, neutrophils, macrophages and proinflammatory cytokines, while increasing anti-inflammatory cytokines in brain tissue (Zhang et al., 2013). Additionally, irradiation of the male spleen 4 hours after MCAO reduces infarct volume by abrogating deployment of splenocytes and decreasing microglia and infiltrating T cells in the ischemic brain (Ostrowski et al., 2012).
In this study, we examine the role of the spleen during stroke in female mice. Male and female mice underwent splenectomy or sham splenectomy two weeks prior to transient MCAO. After 96 hours of reperfusion, infarct volume was determined and peripheral and ischemic brain specific immunological parameters were studied. We found that the sex difference observed in infarct volume after MCAO was abrogated with splenectomy. Activated T cells in the periphery correlated with the sex and splenectomy difference seen in the infarct volume while CD11b+ monocytes were an indicating factor of stroke outcome in male mice. Finally, immune gene expression in the ischemic brain varied, with some genes being directly correlated with infarct volume while others were more affected by splenectomy or sex. The current study provides new insight to how the immune response contributes to sex differences after stroke.
2. Materials and Methods
2.1 Ethics Statement
The study was conducted in accordance with National Institutes of Health guidelines for the use of experimental animals, and the protocols were approved by the Portland Veteran Affairs Medical Center Institutional Animal Care and Use Committee, protocol # 2840-12, local database ID # 2840 and the Oregon Health and Science University Animal Care and Use Committee, protocol # IS00001589.
2.2 Experimental Animals
Male and female C57BL/6J wild-type (WT) mice (The Jackson Laboratory, Sacramento, CA, USA) weighing 20–25 g were housed in a climate-controlled room on a 12-hour light/dark cycle. Food and water were provided ad libitum.
2.3 Splenectomy
Splenectomy was done under isoflurane anesthesia (induction 5.0% and maintenance 2.0% delivered via a face mask in O2-enriched air). A longitudinal incision (10–15mm) was made on the left dorsolateral side of the abdomen, caudal to the last rib, then a 10mm incision in the peritoneal wall was made. The splenic arteries and efferent venous were ligated with sterilized 6-0 silk sutures separately by looping the sutures through the mesentery. The mesentery and connective tissue were cut and the spleen removed. Abdominal muscle incisions and skin were closed using sterilized 6-0 absorbable sutures separately. Sham splenectomized mice underwent all anesthetic and surgical manipulations without handling or removing the spleen. Spleens were removed 14 days before MCAO.
2.4 Middle Cerebral Artery Occlusion Model
Two weeks after splenectomy or sham splenectomy, transient focal cerebral ischemia was induced in male and female mice for 1 hour by reversible right MCAO under isoflurane anesthesia followed by 96 hours of reperfusion as described previously (Zhang et al., 2008). Head and body temperature were controlled at 37.0 ± 1.0°C throughout MCAO surgery with warm water pads and a heating lamp. Occlusion and reperfusion were verified in each animal by laser Doppler flowmetry (LDF) (Model DRT4, Moor Instruments Ltd., Wilmington, DE). The common carotid artery was exposed and the external carotid artery was ligated and cauterized. Unilateral MCAO was accomplished by inserting a 6-0 nylon monofilament surgical suture (ETHICON, Inc., Somerville, NJ, USA) with a heat-rounded and silicone-coated (Xantopren comfort light, Heraeus, Germany) tip into the internal carotid artery via the external carotid artery stump. Adequacy of MCAO was confirmed by monitoring cortical blood flow at the onset of the occlusion with a LDF probe affixed to the skull. Animals were excluded if mean intra-ischemic LDF was greater than 30% pre-ischemic baseline LDF. At 1 hour of occlusion, the occluding filament was withdrawn to allow for reperfusion. Mice were then allowed to recover from anesthesia and survived for 96 hours following initiation of reperfusion. Femoral arterial catheters were placed in separate groups of spleen intact and splenectomized male and female mice (n = 5 per group) to determine mean arterial blood pressure, blood gases (pH, PaO2, and PaCO2), and blood glucose values at 30 min of MCAO (intra-ischemic) and at 15 mins of reperfusion (early reperfusion). Laser Doppler flowmetry was also monitored as described above. Animals were anesthetized with isoflurane throughout ischemia and reperfusion. Head and body temperature were controlled as described above. Mice were euthanized after the final blood sample was taken 15 mins into the reperfusion period.
2.5 Infarct Volume Analysis
Brains were harvested after 96 hours of reperfusion and sliced into five 2-mm-thick coronal sections for staining with 1.2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO, USA) in saline as described previously (Hurn et al., 2007). The 2-mm brain sections were incubated in 1.2% TTC for 15 min at 37°C, and then fixed in 10% formalin for 24 hours. Infarct volume was measured using digital imaging and image analysis software (Systat, Inc., Point Richmond, CA, USA). To control for edema, infarct volume (cortex, striatum, and hemisphere) was determined by subtraction of the ipsilateral noninfarcted regional volume from the contralateral regional volume. This value was then divided by the contralateral regional volume and multiplied by 100 to yield regional infarct volume as a percent of the contralateral region.
2.6 Leukocyte isolation from brains and blood
Blood was obtained from cardiac puncture 96 hours after MCAO. Red blood cells were lysed using 1× red blood cell lysis buffer (eBioscience, Inc., San Diego, CA) for 5 min at room temperature. The cells were then washed with RPMI 1640, counted on a hemocytometer and resuspended in staining medium (PBS containing 0.1% NaN3 and 1% bovine serum albumin (Sigma, Illinois)) for flow cytometry. The brain was divided into the ischemic (right) and nonischemic (left) hemispheres, digested for 60 min with 1 mg/mL Type IV collagenase (Sigma Aldrich, St. Louis, MO) and DNase I (50 mg/mL, Roche Diagnostics, Indianapolis, IN) at 37°C with intermittent shaking. Samples were mixed with a 1 mL pipette every 15 min. The suspension was washed 1× in RPMI, resuspended in 80% Percoll overlayed with 40% Percoll and centrifuged for 30 min at 1600 RPM. The cells were then washed twice with RPMI 1640 and resuspended in staining medium for flow cytometry or stimulation medium for intracellular cytokine detection.
2.7 Analysis of cell populations by flow cytometry
All antibodies were purchased (BD Biosciences, San Jose, CA or eBioscience, Inc., San Diego, CA). Four-color (FITC, PE, APC, PECy5 and/or PerCP) fluorescence flow cytometry analyses were performed to determine the phenotypes of blood and brain cells. 1–2×105 cells were washed with staining medium, blocked with Anti-mouse CD16/CD32 Mouse BD Fc Block™ (BD Biosciences, San Jose) and then incubated with combinations of the following monoclonal antibodies: CD11b (MAC-1), CD45 (Ly-5), CD3 (145-2C11), CD11c (HL-3), CD19 (1D3), CD4 (GK1.5), CD8 (53–6.7), CD122 (TM-β1), CD44 (IM7), CCR5 (HM-CCR5) and CD25 (7D4) for 20 min at 4°C. 7-AAD was added to identify and exclude dead cells in the analysis. CD4+ regulatory T cells were identified using Foxp3 (FJK-16s) and accompanying Fixation/Permeabilization reagents as per manufacturer’s instructions (eBioscience, Inc., San Diego, CA). Isotype matched mAb served as a negative control. Data were collected with BD AccuriTM C6 software on a BD AccuriTM C6 (BD Biosciences, San Jose, CA).
2.8 Intracellular staining
Brain cells from individual mice were cultured at 2×105 cells/well in a 96-well culture plate in stimulation medium (RPMI, 1% sodium pyruvate, 1% L-glutamine, 0.4% 2-β-mercaptoethanol, 2% FBS) with PMA (50 ng/mL), ionomycin (500 ng/mL) and brefeldin A (1 µl/mL) (all reagents from Sigma-Aldrich, St. Louis, MO) for 4 hours at 37°C. Cells were blocked, surface stained (as described above) and then fixed and permeabilized using a Cytofix/Cytoperm kit (BD Biosciences), according to manufacturer’s instructions. Fixed cells were washed with 1× permeablization buffer (BD Biosciences) and stained with the following antibodies: IFN-γ, TNF-a, IL-17 or IL-21. Isotype matched mAbs served as negative controls to establish background cytokine staining levels. Data were collected with BD AccuriTM C6 software on a BD AccuriTM C6 (BD Biosciences, San Jose, CA).
2.9 Real time PCR
Ischemic hemispheres were harvested from mice 96 hours after MCAO, frozen in liquid nitrogen and stored at −80°C until processed. Total RNA was isolated using the RNeasy Mini Kit according to the manufacturer's instructions. (Qiagen, Valencia, CA). cDNA was synthesized using the SuperScript II Reverse Transcriptase cDNA synthesis kit (Life Technologies, Grand Island, NY). Quantitative real time PCR was performed using the StepOnePlus Real-Time PCR System with TaqMan Gene Expression Array Plates for mouse immune response. cDNA from three mice was pooled per group for gene array plates. Individual samples were analyzed in triplicate for IL-6 (Mm00446190_m1), P-selectin (Mm01295931_m1) and IL-10 (Mm00439614_m1) (Life Technologies, Grand Island, NY) using the ABI7000 Sequence Detection System. GAPDH housekeeping gene was used as an endogenous control. Results were analyzed using DataAssist Software (Life Technologies, Grand Island, NY).
2.10 Statistical Analysis
Data are presented as mean ± SEM. Differences in cortical, striatal, and total (hemispheric) infarct volume as well as mean arterial blood pressure, blood gases (pH, PaO2, and PaCO2), and blood glucose were subjected to two-way ANOVA with post hoc Newman-Keuls test. Statistical analyses were performed using SigmaStat Statistical Software, Version 3.1 (SPSS, Inc., Chicago, IL, USA). Cellular subtypes for FACS analysis were analyzed by Student’s t-test using Prism (GraphPad Software, La Jolla, CA). Statistical comparisons were made between spleen-intact males vs. females, splenectomized males vs. females, spleen-intact males vs. splenectomized males and spleen-intact females vs. splenectomized females. Statistical significance was p<0.05.
3. Results
3.1 Splenectomy attenuates sex differences in infarct volume following experimental stroke in mice
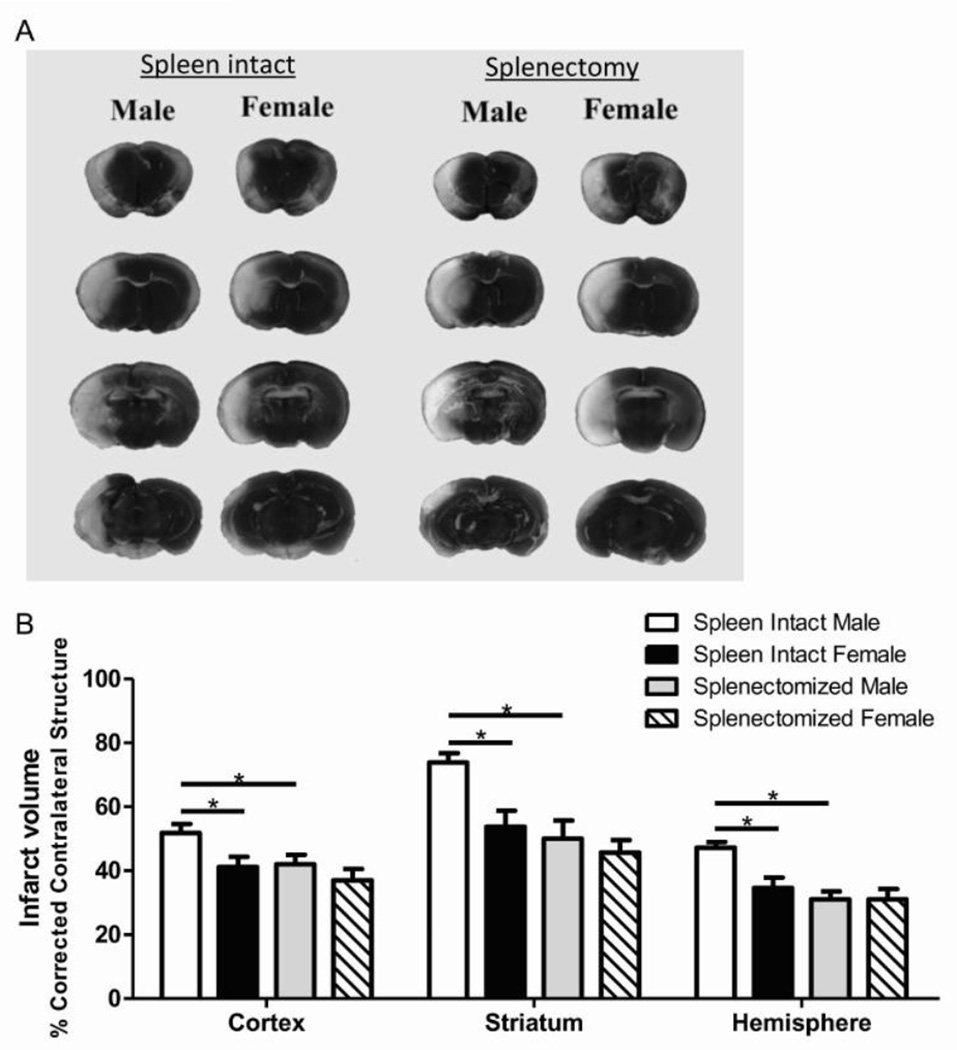
Male and female mice underwent splenectomy or sham splenectomy two weeks prior to 1 hour of MCAO. Infarct volume was measured after 96 hours of reperfusion. In agreement with our recent study (Banerjee et al., 2013), spleen-intact male mice had significantly larger infarct volume than spleen-intact female mice (Fig. 1A). Our findings also support previous studies from other labs (Zhang et al., 2013) indicating that splenectomy significantly reduced infarct volume in male mice (Fig. 1B). Interestingly, splenectomy did not further reduce infarct volume in female mice and there was no difference between splenectomized male and splenectomized female infarct volume (Fig. 1B) indicating that splenectomy abrogates the sex difference observed in brain damage following stroke.
Figure 1.
Splenectomy attenuates sex differences in infarct volume following experimental stroke in mice. Male and female mice were subject to sham splenectomy or splenectomy two weeks prior to transient MCAO (60 min). Brains were harvested 96 h after MCAO and brain slices were stained with 2,3,5-triphenyltetrazolium chloride (TTC) (A). Infarct volumes were measured as percentage of contralateral structure (B). Values represent mean numbers (±SEM) of 11 spleen-intact male and female mice; n=12 for splenectomized males and females. * indicates p<0.05 analyzed using a Two-Way ANOVA.
Mean arterial blood pressure and blood glucose were comparable among male and female spleen intact and splenectomized experimental groups at 30 min of MCAO (Supplemental Table 1). However, there were differences in pH values between splenectomized males (7.48 ± 0.03) and splenectomized females (7.38 ± 0.03, p = 0.043). There were also differences in PaO2 values between spleen intact females (162 ± 5 mm Hg) and splenectomized males (137 ± 7; p = 0.023) and in PaCO2 values between splenectomized males (22 ± 2 mm Hg) and splenectomized females (33 ± 3 mm Hg, p = 0.023) as well as between splenectomized males and spleen intact males (33 ± 1 mm Hg, p = 0.042). Mean arterial blood pressure, blood gases (pH, PaO2, and PaCO2), and blood glucose were comparable among male and female spleen intact and splenectomized experimental groups at 15 min of reperfusion (Supplemental Table 1). There were differences between ischemic and reperfusion pH values within spleen intact males (ischemic: 7.40 ± 0.02; reperfusion: 7.49 ± 0.02; p = 0.009) and splenectomized males (ischemic: 7.48 ± 0.03; reperfusion: 7.41 ± 0.04; p = 0.049).
3.2 Sex differences in peripheral immune frequencies following stroke are CD4+ T cell based
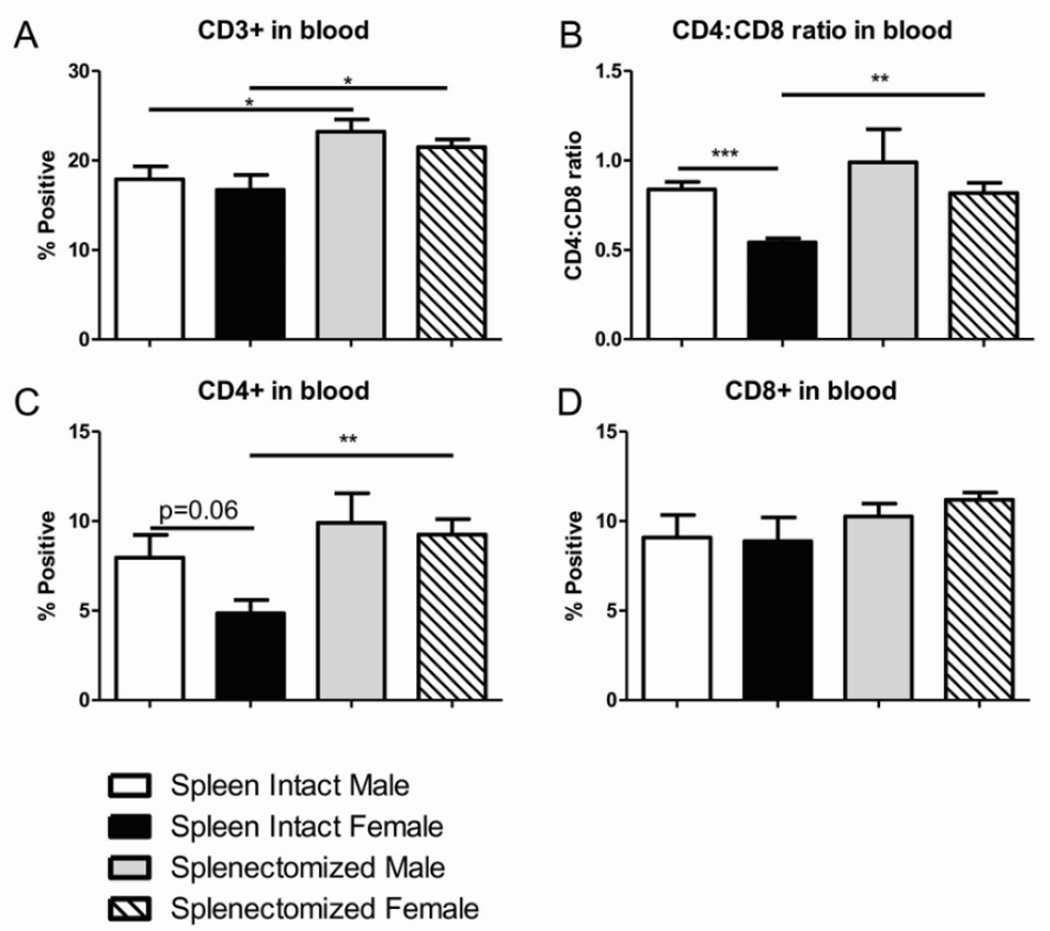
We wanted to identify possible immunological mechanisms in the periphery that could be contributing to the differences in infarct volume we observed with splenectomy and between the sexes. There was no significant difference between males and females, either spleen-intact or splenectomized, in frequency of total T cells in the blood after MCAO (Fig. 2A). Of note, there was an increase in frequency of total CD3+ T cells in the blood after MCAO in splenectomized compared to spleen-intact animals (Fig. 2A). We hypothesize this difference is an artifact of splenectomy, not an effect of stroke. There was a significant difference in the CD4:CD8 ratio in the blood between spleen-intact males and females following MCAO that was not observed with splenectomized males and females (Fig. 2B). The difference in the CD4:CD8 ratio of spleen-intact animals can be attributed to a trending decrease of CD4+ T cells (p=0.06) in the periphery of spleen-intact females after MCAO compared to spleen-intact males (Fig. 2C) as no significant difference was observed in the frequency of CD8+ T cells in the blood with any of groups (Fig. 2D). Additionally, we observed a significant increase in the CD4:CD8 ratio and frequency of CD4+ T cells in the periphery of splenectomized females compared to spleen-intact females following MCAO (Fig. 2B,C) solidifying the hypothesis that peripheral CD4+ T cells are altered in females compared to males after MCAO and that difference is lost with splenectomy.
Figure 2.
Sex differences in peripheral immune frequencies following stroke are CD4+ T cell based. Blood was harvested 96 hours after MCAO from spleen-intact and splenectomized mice and immunophenotyped by flow cytometry. The frequency of CD3+ T cells was determined (A). CD4:CD8 ratio was determined by dividing the frequency of CD4+ cells by the frequency of CD8+ cells (B). CD4 and CD8 T cells were gated on CD3 and the frequency was reported as the percent CD4 or CD8 positive of total CD3+ cells (C,D). Values represent mean numbers (±SEM) of 5 mice from each group for CD4 and CD8 analysis and 8–9 mice per group for CD3 analysis. * indicates p<0.05, ** indicates p <0.01 and *** indicates p<0.001 by t-test.
3.3 Peripheral T cell activation after MCAO is reduced in females and splenectomized males
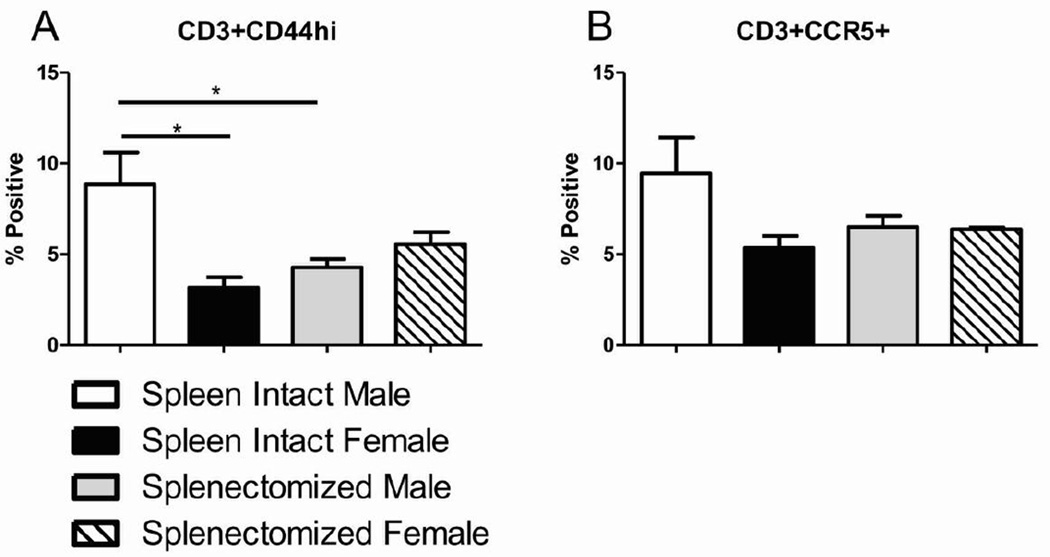
Although we saw no difference between the sexes in the frequency of peripheral T cells after MCAO, we wanted to examine their activation state. We found that CD44hi activated T cells were greater in the periphery of spleen-intact male mice after MCAO compared to either spleen-intact female mice or splenectomized male mice (Fig. 3A). Although not significant, a similar trend was seen with the expression of tissue homing chemokine receptor, CCR5, on T cells following MCAO (Fig. 3B). The sex difference observed in peripheral activated T cells after MCAO supports our hypothesis that that T cell activation in the periphery influences stroke outcome and that sex difference is no longer observed following splenectomy.
Figure 3.
Peripheral T cell activation after MCAO is reduced in females and splenectomized males. Blood was harvested 96 hours after MCAO from spleen-intact and splenectomized mice and immunophenotyped by flow cytometry. The frequency of activated T cells was determined by gating on CD3+ cells and measuring expression of CD44 (A). The frequency of migrating T cells was determined by gating on CD3+ cells and measuring expression of CCR5 (B). Values represent mean numbers (±SEM) of 3–4 mice per group. * indicates p<0.05 by t-test.
3.4 Regulatory T cells in the periphery after MCAO are affected by sex and splenectomy
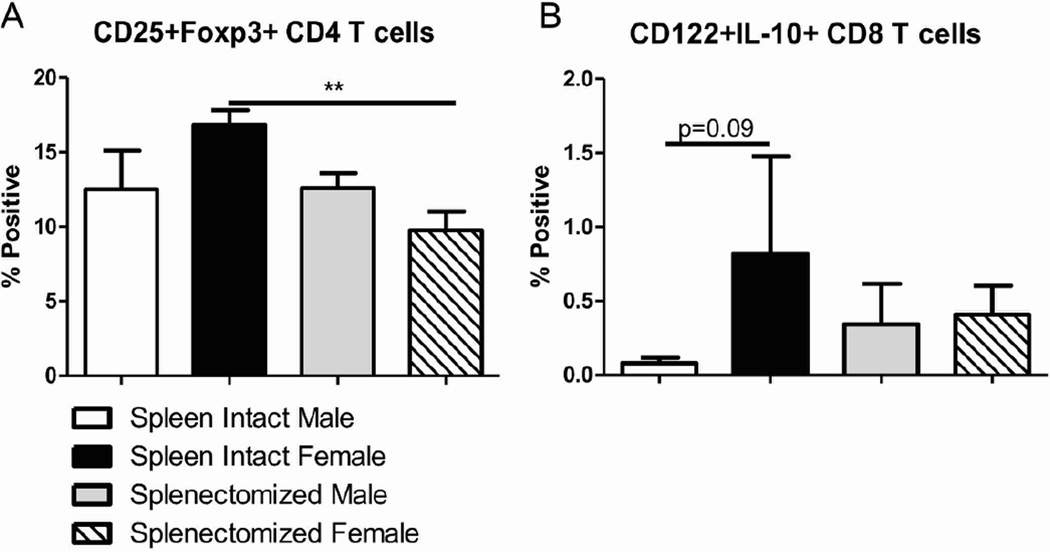
Regulatory T cells have been documented to increase in the periphery following MCAO in male mice (Offner et al., 2006a). Of the four groups of mice, the frequency of CD25+Foxp3+ CD4+ regulatory T cells were the greatest in spleen-intact females after MCAO (Fig. 4A). That frequency significantly dropped in splenectomized females after MCAO and there was no change between spleen-intact and splenectomized males after MCAO (Fig. 4A). The frequency of peripheral CD122+IL-10+CD8+ regulatory T cells was not significantly different among any of the groups following MCAO although spleen-intact females did exhibit a trend increase (p=0.09) in CD8+ regulatory T cells compared to spleen-intact male mice (Fig. 4B). These data suggest that regulatory T cells may have an early protective effect in the periphery of spleen-intact females after MCAO that is no longer necessary in the splenectomized females. Additionally, regulatory T cells may not play a significant role in stroke outcome in male mice.
Figure 4.
Regulatory T cells in the periphery after MCAO are affected by sex and splenectomy. Blood was harvested 96 hours after MCAO from spleen-intact and splenectomized mice and immunophenotyped by flow cytometry. The frequency of CD4+ regulatory T cells was determined by gating on CD4+ cells before measuring CD25, Foxp3 double positive cells (A). The frequency of CD8+ regulatory T cells was determined by gating on CD3+ and CD8+ cells before measuring CD122 and IL-10 expression (B). Values represent mean numbers (±SEM) of 3–6 mice per group with CD4+ Tregs and 2–5 mice per group with CD8+ T regs. ** indicates p<0.01 by t-test.
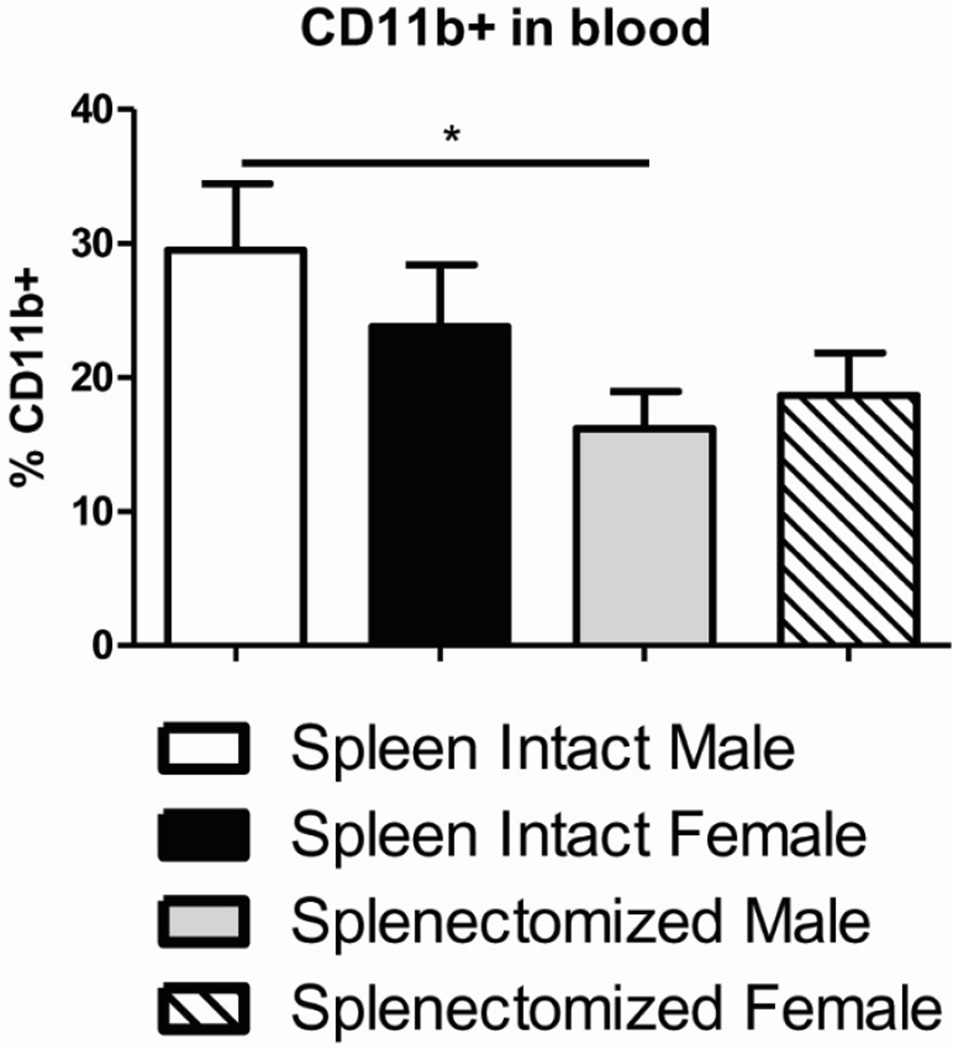
3.5 Peripheral CD11b+ monocytes are reduced in splenectomized male mice after MCAO
We wanted to examine other immune subsets in the periphery that could possibly affect stroke outcome. The frequency of CD19+ B cells did not change among any of the groups following MCAO (Table 1). We did observe significant differences in CD11c+ dendritic cells (DCs) among the groups after MCAO. However, the total frequency of DCs was so low it is doubtful the slight but significant differences could not have any profound effects on stroke outcome (Table 1). There was a significant and substantial reduction in the frequency of CD11b+ monocytes (29.5% to 16.2%) in the blood of splenectomized male mice following MCAO compared to spleen-intact male mice (Fig. 5). These data indicate that peripheral CD11b+ cells are important in stroke outcome in male but possibly not female mice.
Table 1.
Immune subsets in the blood
| Cell type (%) | spleen intact male | spleen intact female | splenectomized male | splenectomized female |
|---|---|---|---|---|
| CD3 | 17.9±1.4 | 16.7±1.7 | 23.2±1.4# | 21.5±0.9# |
| CD4 | 8.0±1.3 | 4.9±0.7 | 9.9±1.7 | 9.2±0.9## |
| CD8 | 9.1±1.3 | 8.9±1.3 | 10.3±0.7 | 11.2±0.4 |
| CD4:CD8 ratio | 0.84±0.04 | 0.54±0.02*** | 0.99±0.2 | 0.82±0.06### |
| CD11c | 0.47±0.03 | 0.35±0.02* | 0.48±0.02 | 0.77±0.06**## |
| CD11b | 29.5±5.0 | 23.8±4.6 | 16.2±2.8# | 18.7±3.1 |
| CD19 | 41.0±7.2 | 49.3±7.9 | 54.3±6.2 | 51.3±5.3 |
indicates significant difference between males and females. *p<0.05; **p<0.01; ***p<0.001
indicates significant difference between spleen intact and splenectomy. #p<0.05; ##p<0.01; ###p<0.001
Figure 5.
Peripheral CD11b+ myeloid cells are reduced in splenectomized male mice after MCAO. Blood was harvested 96 hours after MCAO from spleen-intact and splenectomized mice and immunophenotyped by flow cytometry. The frequency of CD11b+ cells was determined. Values represent mean numbers (±SEM) of 8–10 mice per group. * indicates p<0.05 by t-test.
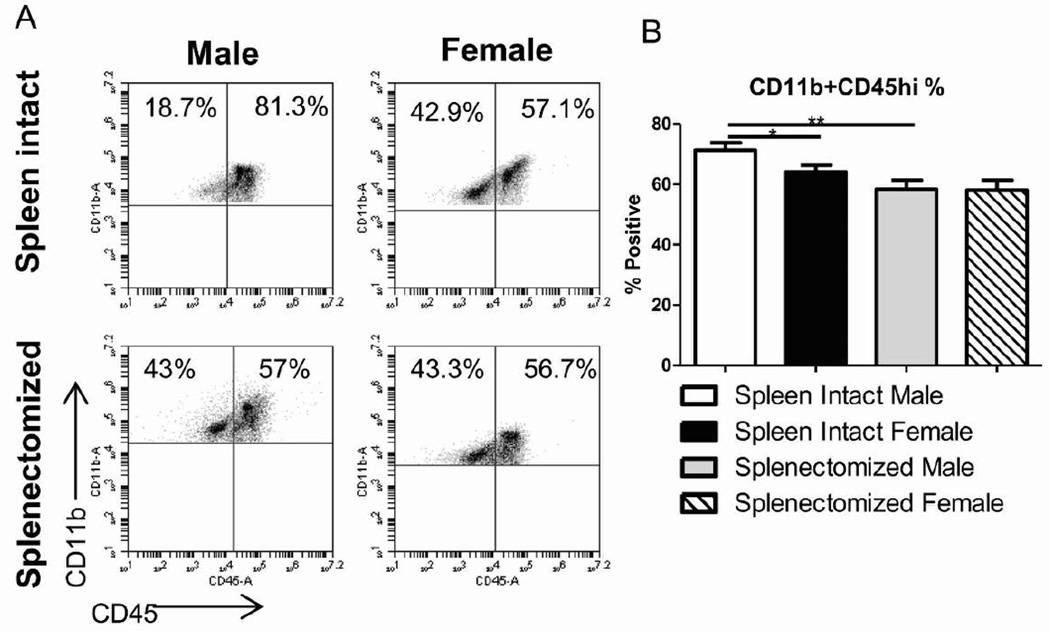
3.6 Splenectomy attenuates sex differences in activated microglia/monocytes in the ischemic brain following experimental stroke
To examine whether sex differences in the peripheral immune response affect tissue specific inflammation, we looked at infiltrating and activated monocytes/microglia in the cerebral ischemic hemisphere. The frequency CD11b+CD45hi infiltrating and activated monocytes/microglia in the ischemic brain was reduced in spleen-intact females, splenectomized males and splenectomized females after MCAO compared to spleen-intact males (Fig. 6A). This difference was significant when comparing infiltrating and activated monocytes/microglia between spleen-intact males and spleen-intact females or splenectomized males after MCAO (Fig. 6B). Concurrent with infarct volume data, there was no significant difference in infiltrating and activated monocytes/microglia between spleen-intact females and splenectomized females or splenectomized males and females following MCAO (Fig. 6B).
Figure 6.
Splenectomy attenuates sex differences in activated microglia/monocytes in the ischemic brain following experimental stroke. Brains were harvested 96 hours after MCAO from spleen-intact and splenectomized mice, processed and immunophenotyped by flow cytometry. Infiltrating and activated monocytes/microglia was determined by gating on CD11b+ cells and examining CD45 high cells. Representative dot plots (A) and averages (B) of CD11b+CD45hi cell frequencies in the ischemic hemisphere are included. Values represent mean numbers (±SEM) of 8–9 mice from each group. * indicates p<0.05, ** indicates p <0.01.
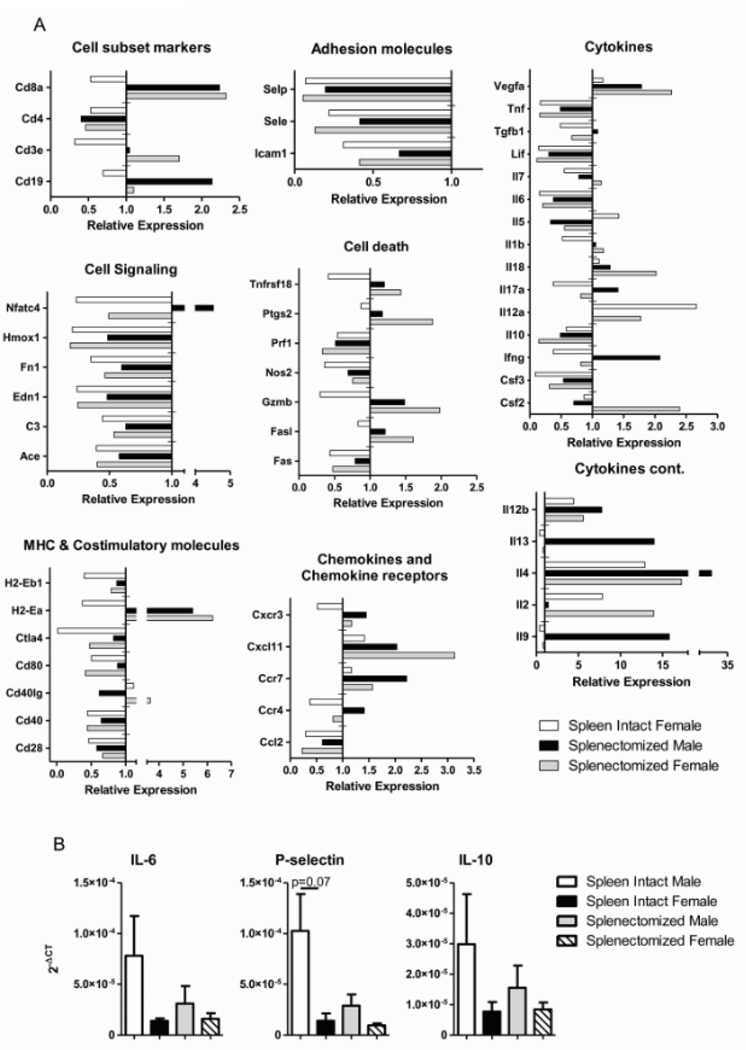
3.7 Sex and splenectomy affect immune gene expression profile in the ischemic brain after MCAO
In order to further assess the mechanism of decreased infarct volume and activated monocytes/microglia with males compared to females and splenectomized animals, we examined immune related genes in the ischemic hemisphere after MCAO using real-time PCR. cDNA was pooled from three samples for each group, results were normalized to GAPDH and presented as relative expression compared to male MCAO only (Fig. 7A). Only parameters that exhibited a two-fold increase or decrease were included in the results. Adhesion molecules and cell signaling genes were largely down-regulated with all groups compared to male MCAO only while cell subset markers, cell death genes, cytokines, costimulatory molecules, chemokines and chemokine receptors exhibited mixed expression patterns (Fig. 7A). Some parameters were affected more by sex such as IL-12a and multiple cell signaling and costimulatory genes while others were affected more with splenectomy, namely, CD8a and H2-Ea expression. Three genes were chosen to validate the immune gene array data with individual samples. IL-6, P-selectin and IL-10 all confirm trends of gene expression observed in the array analysis compared to male MCAO only (Fig. 7B). Cytokine production by specific leukocytes in the ischemic hemisphere additionally follows trends exhibited with the gene expression (Table 2). The data suggests a complex system of gene regulation and expression that is affected by both sex and splenectomy and leads to an overall protection from stroke induced ischemia.
Figure 7.
Gene expression profile from ischemic brain. Relative expression of mRNA immune genes in the ischemic hemisphere 96 hours after MCAO was analyzed by real-time PCR. Samples from 3 mice were pooled per group for analysis and results are relative to the male MCAO only group which was normalized to 1 (A). Values greater than 1 represent upregulated genes and values less than 1 represent down-regulated genes compared to male MCAO only. Gene expression was validated by confirming three genes with unpooled samples in triplicate (B). Values represent mean numbers (±SEM) of 3 mice per group.
Table 2.
Cytokine production in the ischemic hemisphere
| Cell type/Cytokine (absolute number) |
spleen intact male | spleen intact female | splenectomized male | splenectomized female |
|---|---|---|---|---|
| CD4+IL-17+ | 214 ± 81.48 | 126.2 ± 64.9 | 124.4 ± 31.1 | 85.5 ± 37.1 |
| CD4+IL-21+ | 296.2 ± 77.3 | 114.4 ± 44.7 | 189.2 ± 94.1 | 109.6 ± 17.4 |
| CD4+TNF-α+ | 1498 ± 369.9 | 689.7 ± 246 | 838.3 ± 328.7 | 350 ± 37.6 |
| CD11b+TNF-α+ | 8843 ± 3421 | 4952 ± 1435 | 5273 ± 2189 | 1401 ± 336.4 |
| CD4+IFN-γ+ | 53.1 ± 17.2 | 51.6 ± 13.7 | 115.8 ± 26.7 | 83.8 ± 29.3 |
| CD8+IFN-γ+ | 421.2 ± 155.9 | 224 ± 13.4 | 434.7 ± 150.7 | 394.3 ± 61.9 |
4. Discussion
The peripheral and brain-specific inflammatory response to cerebral ischemia has been widely studied, though mainly in male animals. Young female mice exhibit smaller infarct volumes than males following experimental stroke (Alkayed et al., 1998, Banerjee et al., 2013, Manwani et al., 2013). Immunological factors thought to influence the sex stroke difference 96 hours after MCAO are infiltrating and activated monocytes/microglia in the brain and T cell subsets in the periphery (Banerjee et al., 2013). Splenic atrophy, a hallmark of post-stroke peripheral immune activation, is significantly reduced in young female mice after experimental stroke compared to males (Banerjee et al., 2013, Manwani et al., 2013). The comparison of spleen in intact and splenectomized male and female mice allows for a unique investigation into the effects of the peripheral immune response on the different sexes after MCAO.
The current study verifies that infarct volume is smaller in young female mice compared to males and in splenectomized males compared to spleen-intact males following experimental stroke. Additionally we demonstrate that the sex infarct volume difference is eliminated with splenectomy in MCAO mice. Our findings are not explained by differences in physiological parameters among treatment groups. Although differences were seen in pH values between splenectomized males and females during ischemia and between ischemic and reperfusion time points within spleen intact and splenectomized male groups, these values fell within the reported normal reference range for mice. It is therefore unlikely that these differences account for the effect of splenectomy on attenuating sex differences in infarct volume. Differences in arterial O2 tension between intact females and splenectomized males also unlikely affected infarct volumes as both of these groups, along with the other experimental groups, were hyperoxic (>120 mm Hg) but cerebral blood flow has been shown to be relatively unchanged by hyperoxia. Compared to spleen intact males and splenectomized females, splenectomized male mice had significantly lower arterial CO2 tension during ischemia but had comparable values to all experimental groups in the early reperfusion period. However, it is unlikely that the hypocapnia observed in splenectomized male mice during ischemia accounts for the protective effect of splenectomy on infarct volume in males as a relative hypocapnia would decrease cerebral blood flow and thus would have further exacerbated infarct volume. Furthermore, the LDF signal was reduced by a similar percentage of baseline values in all groups, suggesting that relative cerebral blood flow was equivalent among all groups despite any differences in PaO2 or PaCO2 during ischemia. There were no differences in physiological parameters among the experimental groups during the early reperfusion period.
The frequency of peripheral T cells did not differ between both spleen-intact or splenectomized males and females. However, there was a significant difference in the CD4:CD8 ratio in the blood following MCAO between spleen-intact males and females which correlates with CD4+ T cells in the periphery of the male mice. These data are consistent with an increase of CD4+ T cells observed in the spleens of male vs. female MCAO mice (Banerjee et al., 2013). There is also an overall increase in the frequency of peripheral CD3+ T cells in both male and female splenectomized mice compared to spleen-intact mice after MCAO. This increase in T cell numbers could be attributed to the phenomenon of peripheral leukocytosis due to splenectomy (Djaldetti et al., 2003) and did not correlate with an increase in either activated or regulatory T cells, nor did it have a direct effect on CD3+ gene expression in the brain and therefore did not appear to affect stroke outcome.
Among the total T cells in the periphery after MCAO, there were significantly more CD44high expressing effector T cells than in spleen-intact male mice. CD44 is a widely expressed adhesion receptor that is up-regulated on the T cell surface following activation and can lead to cell migration and recruitment to inflammatory sites, thereby exacerbating inflammatory conditions (Johnson and Ruffell, 2009). Since we did not observe a significant increase of CCR5, a chemokine receptor involved in leukocyte homing to sites of inflammation, present on peripheral T cells from spleen-intact males, we speculate that CD44 along with other adhesion molecules are more important for inflammatory cell migration to the brain following stroke. There was also a trend increase in CD4+CD25+Foxp3+ and CD8+CD122+IL-10+ regulatory T cells in the periphery of spleen-intact females after experimental stroke. Although CD4+ regulatory T cells have an established role in suppressing inflammation, there are contradicting reports whether they are beneficial, harmful or have no effect on neurodegeneration following stroke (Schabitz, 2013, Liesz et al., 2009, Planas and Chamorro, 2009, Li et al., 2013, Kleinschnitz et al., 2013a, Kleinschnitz and Wiendl, 2013b, Ren et al., 2011, Gu et al., 2012, Stubbe et al., 2013, Xu et al., 2013). CD8+ regulatory T cells regulate immune effector function using perforin and IL-10 (Wang and Alexander, 2009) and have been observed in conjunction with neuroprotection following stroke (Banerjee et al., 2013, Bodhankar et al., 2013). Although we observe a similar trend in CD8+ regulatory T cells as with CD4+ regulatory cells from spleen-intact females, it is unclear whether such a small percentage of IL-10 producing cells in the periphery could have an effect on infarct volume. Taken together, the dynamic and inverse shift between effector and regulatory T cell phenotype suggests that regulatory T cells may have a protective effect in the periphery of spleen-intact females after MCAO compared to spleen-intact males. That difference is lost or no longer necessary in the splenectomized mice. Additionally, since splenectomy did not have an effect on CD4+ regulatory T cells in male mice, it appears not to play a significant role in stroke outcome in males.
Experimental stroke leads to an increase in circulating CD11b+ monocytes (Offner et al., 2006b). Activated monocytes and macrophages secrete inflammatory cytokines such as IL-1β, IL-6 and TNFα which can perpetuate the inflammatory state and lead to tissue specific monocyte homing. Spleen-intact male mice exhibited a significant and sizable increase in circulating CD11b+ monocytes compared to splenectomized male mice following MCAO. The same difference was not observed between spleen-intact and splenectomized female mice. Additionally, there was no significant sex difference in the frequency of blood CD11b+ monocytes with spleen-intact animals, suggesting that peripheral CD11b+ monocytes may be important for neurodegenerative progression in males and possibly do not have as much of a detrimental effect in females.
It is known that the spleen exacerbates brain inflammation after stroke and splenectomy prior to experimental stoke decreases infarct volume through anti-inflammatory effects (Seifert et al., 2012b, Zhang et al., 2013). To determine if the effects of the spleen on brain inflammation differed between males and females, we first looked at infiltrating and activated monocytes/microglia in the ischemic hemisphere with and without splenectomy. As expected, the number of activated monocytes/microglia correlated directly with infarct volume after MCAO, with a significant decrease in spleen-intact females and splenectomized males compared with spleen-intact males. One factor that contributes to the sex difference observed in activated monocytes/microglia is estrogen. Estrogen inhibits the proliferation of microglia in vitro, attenuates macrophage cytokine production and can reduce the monocyte infiltration to the ischemic brain by dampening migration and adhesive capabilities (Ritzel et al., 2013). Additionally, no difference was observed between spleen-intact and splenectomized females.
To further examine the mechanism in the brain, we looked at differences in immune gene expression in the ischemic hemisphere. Spleen-intact female mice had decreased CD3, CD4 and CD8 expression after MCAO, while splenectomized mice varied in CD3 expression but downregulated CD4 and upregulated CD8 expression compared to male MCAO only mice. Although the increase in CD8 did not correlate with IL-10 production, indicating CD8+Tregs, the elevation in CD8 did not increase neurodegeneration. Adhesion molecules are known to play an important role in leukocyte infiltration into the brain after experimental stroke and their blockade results in reduced infarct volume (Yilmaz and Granger, 2008, Zhang et al., 1998). Cell signal genes have also been shown to affect stroke outcome (Ziv et al., 1992, Yusuf et al., 2000). Overall, there was a substantial decrease of adhesion molecule and cell signaling expression with all groups compared to spleen-intact males after MCAO, albeit more reduced with the two female groups, which could account for neuroprotection observed in those groups. Inflammatory cytokines are known to contribute to neuroinflammation after stroke (Seifert et al., 2012b, Barone et al., 1997, Feuerstein et al., 1994, Bond et al., 2002, Slevin et al., 2008). We observed a decrease in gene expression in all the mouse groups for TNF, IL-6 and Lif and to a lesser extent, IL-17 and IFNγ in females compared to spleen-intact males after MCAO.
This study demonstrates that the peripheral immune response contributes to the infarct volume-associated sex difference in experimental stroke. Both sex and splenectomy reduce activated T cells in the periphery after MCAO, while CD11b+ monocytes were affected more by splenectomy than by sex. In the ischemic brain, sex and splenectomy influenced activated monocytes/microglia and expression of adhesion molecules, cell signaling molecules and specific inflammatory cytokines. Taken together, these data provide new information about the sex-specific mechanisms of the peripheral immune response in neurodegeneration after stroke. Additionally, this study demonstrates the significant need for representation of both males and females in biological and clinical stroke research.
Supplementary Material
Highlights.
The peripheral immune effects on stroke are compared between male and female mice.
Male and female mice underwent splenectomy or sham splenectomy prior to MCAO.
Splenectomy reduced infarct and activated microglia in males but not females.
Splenectomy eliminated peripheral immune sex difference.
We demonstrate the need for representation of both sexes in stroke research.
Acknowledgements
The authors wish to thank Gail Kent for assistance with manuscript submission. This work was supported by NIH Grant # NS076013 and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations
- MCAO
middle cerebral artery occlusion
- Treg
regulatory T cell
- MHC
Major Histocompatibility Complex
- LDF
Laser Doppler flowmetry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res. 2013;4:554–563. doi: 10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. PD-L1 enhances CNS inflammation and infarct volume following experimental stroke in mice in opposition to PD-1. J Neuroinflammation. 2013;10:111. doi: 10.1186/1742-2094-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond BC, Virley DJ, Cairns NJ, Hunter AJ, Moore GB, Moss SJ, Mudge AW, Walsh FS, Jazin E, Preece P. The quantification of gene expression in an animal model of brain ischaemia using TaqMan real-time RT-PCR. Brain Res Mol Brain Res. 2002;106:101–116. doi: 10.1016/s0169-328x(02)00417-5. [DOI] [PubMed] [Google Scholar]
- Di carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- Djaldetti M, Bergman M, Salman H, Cohen AM, Fibach E, Bessler H. On the mechanism of post-splenectomy leukocytosis in mice. Eur J Clin Invest. 2003;33:811–817. doi: 10.1046/j.1365-2362.2003.01197.x. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev. 1994;6:341–360. [PubMed] [Google Scholar]
- Gu L, Xiong X, Zhang H, Xu B, Steinberg GK, Zhao H. Distinctive effects of T cell subsets in neuronal injury induced by cocultured splenocytes in vitro and by in vivo stroke in mice. Stroke. 2012;43:1941–1946. doi: 10.1161/STROKEAHA.112.656611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8:208–220. doi: 10.2174/187152809788680994. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K, Schuhmann MK, Langhauser F, Helluy X, Schwarz T, Bittner S, Mayer CT, Brede M, Varallyay C, Pham M, Bendszus M, Jakob P, Magnus T, Meuth SG, Iwakura Y, Zernecke A, Sparwasser T, Nieswandt B, Stoll G, Wiendl H. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013a;121:679–691. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Wiendl H. Con: Regulatory T cells are protective in ischemic stroke. Stroke. 2013b;44:e87–e88. doi: 10.1161/STROKEAHA.113.001268. [DOI] [PubMed] [Google Scholar]
- Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, Liang W, Thomson AW, Chen J, Hu X. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. 2013;74:458–471. doi: 10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Suri-payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, Mccullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SJ, Mccullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. Ilar j. 2004;45:147–159. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006a;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006b;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Schulte RW, Nie Y, Ling T, Lee T, Manaenko A, Gridley DS, Zhang JH. Acute splenic irradiation reduces brain injury in the rat focal ischemic stroke model. Transl Stroke Res. 2012;3:473–481. doi: 10.1007/s12975-012-0206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas AM, Chamorro A. Regulatory T cells protect the brain after stroke. Nat Med. 2009;15:138–139. doi: 10.1038/nm0209-138. [DOI] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2011;26:87–90. doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Capozzi LA, Mccullough LD. Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke. Horm Behav. 2013;63:238–253. doi: 10.1016/j.yhbeh.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabitz WR. Regulatory T cells in ischemic stroke: helpful or hazardous? Stroke. 2013;44:e84. doi: 10.1161/STROKEAHA.113.002228. [DOI] [PubMed] [Google Scholar]
- Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012a;7:1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Leonardo CC, Hall AA, Rowe DD, Collier LA, Benkovic SA, Willing AE, Pennypacker KR. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis. 2012b;27:131–141. doi: 10.1007/s11011-012-9283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Mitsios N, Perikleous C, Cuadrado E, Montaner J, Sanfeliu C, Luque A, Kumar S, Kumar P, Gaffney J. Leukaemia inhibitory factor is overexpressed by ischaemic brain tissue concomitant with reduced plasma expression following acute stroke. Eur J Neurol. 2008;15:29–37. doi: 10.1111/j.1468-1331.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- Stubbe T, Ebner F, Richter D, Engel O, Klehmet J, Royl G, Meisel A, Nitsch R, Meisel C, Brandt C. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab. 2013;33:37–47. doi: 10.1038/jcbfm.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- Wang YM, Alexander SI. CD8 regulatory T cells: what's old is now new. Immunol Cell Biol. 2009;87:192–193. doi: 10.1038/icb.2009.8. [DOI] [PubMed] [Google Scholar]
- Xu X, Li M, Jiang Y. The paradox role of regulatory T cells in ischemic stroke. Scientific World Journal. 2013;2013:174373. doi: 10.1155/2013/174373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- Zhang BJ, Men XJ, Lu ZQ, Li HY, Qiu W, Hu XQ. Splenectomy protects experimental rats from cerebral damage after stroke due to anti-inflammatory effects. Chin Med J (Engl) 2013;126:2354–2360. [PubMed] [Google Scholar]
- Zhang R, Chopp M, Zhang Z, Jiang N, Powers C. The expression of P- and E-selectins in three models of middle cerebral artery occlusion. Brain Res. 1998;785:207–214. doi: 10.1016/s0006-8993(97)01343-7. [DOI] [PubMed] [Google Scholar]
- Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, Debarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv I, Fleminger G, Djaldetti R, Achiron A, Melamed E, Sokolovsky M. Increased plasma endothelin-1 in acute ischemic stroke. Stroke. 1992;23:1014–1016. doi: 10.1161/01.str.23.7.1014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.