Abstract
Background
Buprenorphine opioid agonist treatment (OAT) has established efficacy for treating opioid dependency but early relapse rates are high and are often associated with withdrawal-related or emotional distress.
Methods
To determine whether a novel distress tolerance (DT) intervention during buprenorphine initiation decreases opioid relapse, we conducted a preliminary randomized controlled trial with opioid-dependent outpatients. Participants received buprenorphine-naloxone induction and 3-months of maintenance buprenorphine plus seven, 50-minute manualized, individual sessions (DT vs. Health Education (HE) control) over a 28-day period, linked to clinician medication dosing visits, and beginning 2 days prior to buprenorphine induction. Primary outcomes included use of illicit opioids (positive defined as any self-reported use in the prior 28 days or detected by urine toxicology) and treatment drop out.
Results
Among 49 participants, the mean age was 41 years, 65.3% were male. Persons randomized to DT had lower rates of opioid use at all three monthly assessments, and at 3-months, 72% of HE participants were opioid positive compared with 62.5% of DT participants. Rates of dropout were 24% and 25% in the HE and DT arms, respectively.
Conclusions
This distress tolerance treatment produced a small, but not statistically significant reduction in opioid use during the first three months of treatment although no differences were found in drop-out rates between conditions. If replicated in a larger study, DT could offer clinicians a useful behavioral treatment to complement the effects of buprenorphine. Trial registered at clinicaltrials.org. Trial number NCT01556087.
Keywords: Buprenorphine, distress, relapse, opioid
1. INTRODUCTION
The nonmedical use of opioids, including prescription pain relievers and heroin, is a growing public health concern. In 2011, an estimated 2.2 million Americans met DSM-IV (APA, 1994) criteria for opioid abuse or dependence (Substance Abuse and Mental Health Services Administration, 2012). Buprenorphine, an opioid agonist treatment (OAT) that has been prescribed to more than a million individuals in the U.S. since it became available in 2003 (Boothby and Doering, 2007). Offered as an office-based maintenance treatment alternative to methadone, buprenorphine has demonstrated efficacy in reducing cravings, ameliorating withdrawal discomfort, and increasing periods of abstinence. In regard to treatment effectiveness (variously defined as reducing street crime, illicit drug use, HIV risk, or improving vocational development and psychological functioning), buprenorphine has long-term positive outcomes (Mattick et al., 2008). However, buprenorphine treatment drop-out rates are high, with observational studies reporting 50-65% retention rates at 6 months, and the great majority of attrition occurring during the first three months of treatment (Cunningham et al., 2008; Finch et al., 2007; Fudala et al., 2003; Lee et al., 2009; Magura et al., 2007; Mintzer et al., 2007; Soeffing et al., 2009; Stein et al., 2005).
Lapse to opioid use soon after initiation of buprenorphine is common and a strong predictor of poor treatment retention and return to chronic opioid use. Evidence from our group suggests that a significant proportion of persons initiating buprenorphine will lapse within the first week of treatment and those with a positive opioid toxicology by week four are at five times higher risk for continuing opioid use during treatment, treatment drop-out, and relapse (Stein et al., 2010). In subsequent work, we demonstrated that opioid craving, particularly during the first two weeks of buprenorphine treatment, similarly portends worse treatment outcome (Tsui et al., 2014). Thus, convergent evidence indicates that early craving and lapses to opioid use are both frequent and highly predictive of continued opioid use during buprenorphine treatment and of subsequent relapse (Stein et al., 2005, 2010).
Reasons for early attrition from buprenorphine treatment include inadequate dosing of buprenorphine, a desire to continue illicit drug use, social pressures due to a partner’s or friend’s drug use, and barriers to ongoing medication receipt such as cost and difficulty keeping medical appointments (Gryczynski et al., 2014; Mattick et al., 2008; Stein et al., 2005). However, these factors do not account for all instances of relapse. Early illicit opioid lapse despite motivation for abstinence and pharmacologic treatment of acute withdrawal with buprenorphine implicates the substantial role that early events or situations play in increasing craving and motivating drug-seeking behavior (Goldstein and Volkow, 2002; Lubman et al., 2004; Robinson and Berridge, 2001). Indeed, early recovery situations and events that are associated with emotional distress, including the sensation of inadequate opioid substitution and prolonged withdrawal symptoms, continued exposure to environmental drug cues, stresses of everyday life (e.g. financial, familial), or concurrent mood disorder symptoms, reliably induce craving among treated opioid users (Hyman et al., 2007). Indeed, negative affect is well-established as a primary precipitant of early lapse and features prominently in current models of addiction maintenance and relapse (e.g., Baker et al., 2004; Hendershot et al., 2011; Witkiewitz and Marlatt, 2004), which have informed the development of behavioral treatments unrelated to opioid agonist treatment. Skills for the management and reduction of negative affect (e.g., stress management techniques, avoidance of triggers) are primary elements of these treatments. Meta-analyses indicate that cognitive-behavioral intervention for the treatment for substance use disorder is efficacious (Magill and Ray, 2009). However, recent trials evaluating cognitive-behavioral treatment (Fiellin et al., 2013; Ling et al., 2013) and additional drug counseling (Weiss et al., 2014) have not shown significant benefit over physician management for patients taking buprenorphine (Amato et al., 2011). But earlier studies have not initiated behavioral treatment prior to buprenorphine initiation in preparation for the increased challenge of opioid withdrawal and the risk of early lapse.
Research in the area of nicotine dependence has revealed that it is not solely the severity or intensity of distress, but also one’s ability to tolerate both physical and psychological distress (i.e., distress tolerance) occurring in the context of withdrawal and early abstinence that predicts whether one succumbs to a lapse (Brandon et al., 2003; Brown et al., 2002, 2009; Quinn et al., 1996). In these studies, distress tolerance was measured as duration of persistence on psychological and physical challenge tasks that served as analogs for the types of stresses experienced during nicotine withdrawal.
Like nicotine withdrawal and craving, acute opioid withdrawal, which is required as part of standard clinical care in the hours prior to initiating buprenorphine, and craving in the days and weeks after the initiation of buprenorphine, produce uncomfortable interoceptive symptoms such as bone and muscle aches, restlessness, and nausea. Such experiential discomfort, to a greater or lesser degree, demands the use of distress tolerance skills in order to be successful in maintaining abstinence. Evidence suggests that opioid users are overly sensitive to the discomfort associated with anxiety symptoms (Lejuez et al., 2006; Tull et al., 2007). It seems likely then, that for those opioid users initiating buprenorphine treatment who have a low threshold for tolerating distress, and/or difficulty controlling, avoiding, or suppressing the experience of distress, ongoing illicit drug use may serve as a way to manage discomfort. These illicit substance-based efforts to avoid or escape distress are maintained by negatively reinforcing effects such as the reduction of urges or negative affect, even as euphoria is blocked by the agonist properties of buprenorphine.
Supporting this hypothesis, we have recently extended findings from research on nicotine dependence to opioid dependence. The pattern of lower persistence times on the PASAT (Paced Auditory Serial Addiction Task; Diehr et al., 1998; Holdwick and Wingenfeld, 1999) showed that the probability of opioid lapse was greatest soon after initiating buprenorphine, stabilized over subsequent weeks, and was highest among those with low persistence scores (Strong et al., 2012). Given that inability or reduced ability to tolerate distress interferes with efforts to establish longer-term opioid-free behavior change (Strong et al., 2012), individuals who are initiating buprenorphine treatment may benefit from learning new skills or strategies to tolerate these withdrawal symptoms, cravings, and negative affect during early abstinence.
In the current study, we present an adaptation of a distress tolerance treatment (DT), originally developed for smokers (Brown et al., 2008, 2013), that was tailored for opioid dependent individuals initiating buprenorphine treatment. This treatment combines behavioral exposure to opioid craving with training in skills based in Acceptance and Commitment Therapy (Hayes, 2006) to promote maintenance of abstinence. By teaching buprenorphine initiators to minimize avoidance or efforts to escape this discomfort, treatment is meant to strengthen their ability to remain opioid abstinent. We have reported on the development piloting of a novel DT treatment for buprenorphine initiators previously (Brown et al., 2014). Based on feedback from this open trial work, we have created a treatment that we now test in a preliminary randomized trial comparing the distress tolerance treatment (DT) to a health education (HE) comparison condition.
2. METHODS
2.1 Participants
Participants were recruited via advertisements (newspaper, bus). Inclusion criteria were age 18-65, seeking buprenorphine treatment, and planning to remain on buprenorphine for at least 3 months. Individuals were excluded for: current participation in methadone maintenance treatment; 15 or more days of benzodiazepine or cocaine use in the last month; daily alcohol use or binges weekly or more; medically necessary opioid treatment for chronic pain; surgery in the next 3 months; current suicidality; neuropsychological dysfunction; justice system involvement that might interfere with participation; bipolar or psychotic disorder; or pregnancy.
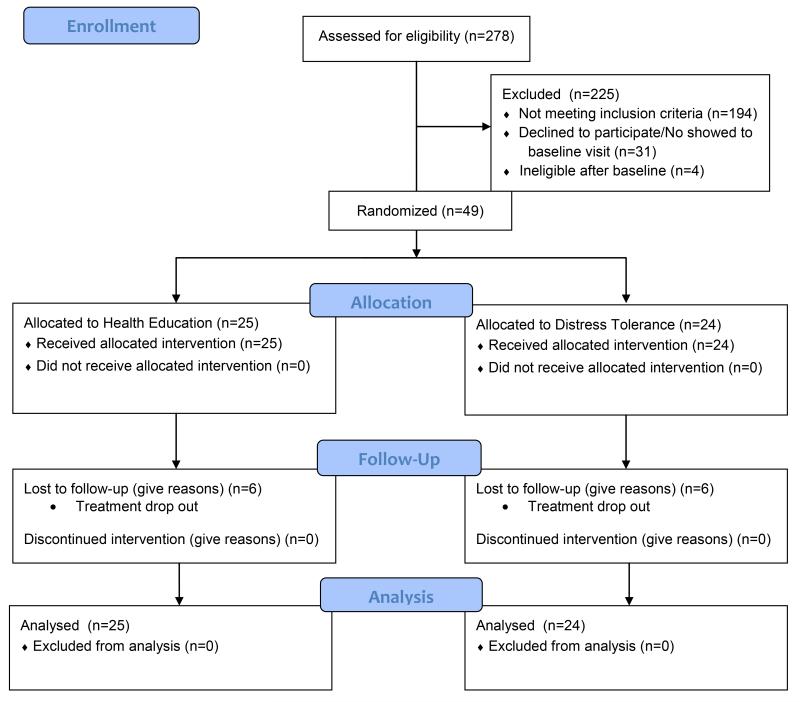
Between April, 2013 and October, 2013, 278 individuals were screened after calling the study line. Of these, 194 did not meet eligibility criteria for the following reasons: 65 reported bipolar symptoms or disorder, 52 had a history of psychotic symptoms, 28 had high levels of benzodiazepine, cocaine, or binge alcohol use, 21 had suicidal ideation, 17 were leaving the area in the next 3 months, and 11 were receiving methadone. Of the 84 eligible persons invited for an interview, 31 did not show up and 53 completed a baseline interview. Four were ruled ineligible (3 due to buprenorphine use and negative opioid toxicology and one due to heavy alcohol). Of the remaining 49 persons, 25 were assigned to health education (HE) and 24 to DT condition using permuted block (block sizes of 4 or 6) randomization generated by an off-site statistician.
2.2 Standard Buprenorphine Care
The office-based buprenorphine-naloxone (hereafter buprenorphine) treatment protocol included an induction day (Day 0), 12 weeks of buprenorphine maintenance treatment, and four additional weeks of buprenorphine taper. At the induction visit, under supervision, four milligrams of buprenorphine (with 1 mg of naloxone, provided as Suboxone tablets) was given sublingually to participants while they were experiencing mild-moderate opioid withdrawal (Wesson and Ling, 2003). Additional buprenorphine was taken home for use later in the day (Lee, et al., 2009) and the next day, with a total dose of 16 milligrams per day. The participant was scheduled to return two days later and given enough buprenorphine to last until the next visit five days later. Physician follow-up visits, paired with blinded assessments, were scheduled for days 7 and 14 after induction and at weeks 4, 6, 8 and 12 (the final study outcome assessment). At each physician visit, participants were given enough buprenorphine to use 16 mg per day until the subsequent appointment. After 12 weeks, the four-week buprenorphine taper began for participants unable to find a long-term provider. Participants planning to continue with another buprenorphine provider were maintained at 16mg, rather than tapering, until they transferred care at week 16. At each visit beginning at induction, brief physician management (PM) counseling was provided by either of the two study clinicians (Fiellin et al., 2006, 2002). Additionally, phone numbers for self-help groups (all off-site) were offered. Twelve-step involvement was recommended but not mandated.
2.3 DT Treatment
The DT treatment (Brown et al., 2014) was drawn from exposure-based and acceptance-based (ACT; Hayes et al., 1999) treatment approaches and adapted from our DT smoking cessation treatment (Brown et al., 2008). In order for an exposure treatment to be maximally effective, individuals must fully engage in exposure without attempts to use distraction or engage in avoidance strategies. Patients need to demonstrate a willingness to remain in this uncomfortable state as they work toward their desired goal of quitting opioids. To this end, ACT strategies were incorporated into the treatment, as ACT places a central focus on acceptance. ACT (Hayes et al., 1999) is designed to produce acceptance behaviors aimed at private events that have interfered with behaving consistently with one’s life values. Acceptance may be defined as actively engaging in the process of experiencing thoughts and feelings without attempting to avoid or change these experiences (Hayes et al., 1999), as well as the behavior of approaching psychologically aversive internal stimuli while behaving adaptively (Gifford, 1994; Gifford and Hayes, 1997).
The DT treatment included seven, 40-50-minute, manualized, individual sessions over a 28-day period, with sessions occurring 1-2 days prior to buprenorphine induction, on induction day (Day 0), on the day after induction, and on days 5, 7, 14, and 28 after induction. Thus, we front-loaded the DT sessions such that the majority occurred prior to buprenorphine initiation and during the first weeks of treatment when the risk of lapse is highest (Stein et al., 2010; Tsui et al., 2014). For pragmatic reasons, and to reduce participant burden, we linked all DT and HE sessions to physician visits.
2.3.1 Sessions 1 and 2 (Day −2 and Day 0 – Buprenorphine Induction)
These first two sessions occurred prior to buprenorphine induction, the first two days prior to induction and the second just hours before induction when the participant was just starting to experience withdrawal symptoms. In Session 1, the initial discussion focused on life values and goals, which allowed a therapeutic alliance to develop and lay the framework for the subsequent sessions. The participant’s list of values was written on an index card (“values card”) so that participants could have a tangible reminder of values that might motivate distress tolerance. After therapists presented the theoretical model of drug use (physical addiction, learned habit, comfort regulation) they introduced the concept of a trigger and the distinction between external (e.g., drug cues, interpersonal conflict) vs. internal (e.g., affective, cognitive, physiological) triggers and personalized for each participant, which lay the framework for later discussions. In Session 2, because participants were experiencing sensations of opioid withdrawal in preparation for buprenorphine induction, the focus was on exposure to withdrawal discomfort, and included meditation exercises, the introduction of the concept of acceptance as a strategy for managing internal triggers, and included the ACT metaphor Man in the Hole (Hayes et al., 1999), which compares efforts to control discomfort to trying to dig one’s way out of a hole as a way to highlight the futility of managing discomfort through continued illicit drug use.
2.3.2 Sessions 3, 4, and 5 (Days 1, 4, and 7)
These sessions during the first week of buprenorphine treatment involved the use of self-management skills for external triggers, (avoiding, altering, delaying or substituting triggers), and acceptance and exposure for internal triggers (through exercises and metaphors). For example, to illustrate the difficulty in controlling or avoiding thoughts, participants were asked what would happen if they were told, “Don’t think about a jelly donut.” They were asked to consider how avoiding this thought would be similar to trying to avoid thoughts about drug use. Through the Quicksand metaphor (Luoma et al., 2007), therapists illustrated how efforts to control or avoid internal experience may actually have the opposite effect, resulting in opioid use and difficulty abstaining.
Willingness was introduced using the Two Scales metaphor (Hayes et al., 1999) – the two scales referring to 1) intensity of discomfort and 2) degree of willingness to experience discomfort without trying to change, avoid, or escape it. It was suggested to participants that although they may try to control the discomfort scale, willingness is the only scale they actually have control over and therefore they should shift their focus to keeping the willingness scale set to maximum (willingness). As part of this component, participants explored their tendency to place limits on acceptance; for example, by making deals or bargains with themselves that they would be willing to endure discomfort only to a certain point, beyond which time they would lapse to drug use (e.g., “If I have a really bad day, then it’s ok to use.”).
Finally, participants used exercises designed to facilitate values clarification and engagement in values-consistent behavior. For example, they imagined themselves in 6 weeks, 3 months, 6 months, and a year as drug-free. We also used an exercise that was adapted from a eulogy exercise (Hayes et al., 1999). In this exercise, participants imagined being contacted 10 years in the future by a publisher who wanted to write their life story, including interviews with the people closest to them. They envisioned what they would ideally want these people to say about how they lived their life.
2.3.3 Sessions 6 and 7 (Days 14 and 28)
The last two sessions occurred two weeks and four weeks after buprenorphine induction. The focus was on providing additional pportunities for exposure and practice of acceptance, willingness, and guiding the participant to create plans to: maintain a commitment to effective changes, develop and maintain social support, prepare relapse prevention strategies, and taper off buprenorphine or transition their care.
All DT sessions began with an interventionist-led five-minute mindfulness meditation exercise to foster present-moment awareness and acceptance-based skills. Each of the 7 sessions closed with the question “What’s one thing you can take away from this lesson that might be helpful to you?” to better understand which concepts, metaphors and skills participants found most relevant and to help them consolidate plans for moving forward. As participants left each session, we encouraged them to review their values cards, and commit to at least one action that would help them get one step closer to living a life that is consistent with their expressed values and goals.
2.4 Health Education Control Condition
We used a Health Education (HE) intervention as a control condition. The seven, 20-30-minute individual information and education sessions were identical in their timing to the DT condition. The seven sessions covered: 1) nutrition, 2) sleep hygiene, 3) building immunity (e.g., how to avoid colds/flu), 4) relaxation strategies (e.g., progressive muscle relaxation, deep breathing etc.), 5) injury/disease prevention (e.g., seat belts), 6) the value of exercise/cardiac health, and 7) alternative medicine (e.g. acupuncture, massage). These sessions were purely didactic and consisted of health education, followed by discussion.
2.5 Therapist Training and Treatment Fidelity
The two therapists, a masters level R.N. and a post-doctoral clinical psychologist who had previous experience with ACT treatment, but had not worked with opioid dependent persons, met with Drs. Herman and Brown weekly to review audiotaped sessions and therapist adherence to the DT and HE manuals. We created manual adherence scales that included all the main elements of each section of the manual, such as the theory behind the treatment, metaphors used, and skill-building exercises, or, for the HE sessions, elements related to each of the didactic information points. Each point of adherence could be rated as Fully Adherent, Partially Adherent, or Not Adherent.
Two clinical psychologists independent of this study each rated a set of audiotaped sessions (12% of DT sessions, 8% of HE sessions) using the study Adherence Measure. DT sessions received consistent ratings 77.3% of the time with average rater ICC = .78. HE sessions received consistent ratings 87.5% of the time with average rater ICC = .74. For the DT sessions, therapists were fully adherent 96.2% of the time, and partially adherent 3.8% of the time. Therapists were fully adherent to the HE sessions 100% of the time, with no evidence of DT elements present. Participants assigned to DT participated in an average of 5.6 sessions, those assigned to HE participated in an average of 6.2 sessions. The average length of the DT sessions was 46 minutes, and of the HE sessions was 24 minutes. Both therapists conducted the DT and HE interventions.
2.6 Measures
Sample descriptors included age, gender, race or ethnicity, ever prescribed buprenorphine (yes/no). The Timeline Followback (TLFB) method (Fals-Stewart et al., 2000) was used at each assessment to assess self-reported drug consumption since the previous assessment. Urine toxicologic testing for opioids including morphine, heroin, oxycodone, and methadone was done at each assessment and physician visit.
2.7 Analytical Methods
We present descriptive statistics to summarize the characteristics of the cohort and compare intervention arms. Because this is a pilot project with a small sample size we have chosen to present measures of effect size and 95% confidence interval estimates. For our primary outcome (any self-reported illicit opioid use in the last 28 days or urine toxicology positive for opioids at each monthly assessment), we present counts and percentages. We also present the between group difference in percent (calculated as the percentage in the HE arm – the percentage in the DT arm) and the 95% confidence interval estimate for that difference. Additionally, we report the population averaged odds-ratio, estimated by GEE, comparing DT to HE across time (Hardin and Hilbe, 2002). The GEE model included baseline frequency of self-reported illicit opioid use and time as covariates. Statistical comparisons of self-reported frequency of illicit opioid use during follow-up were problematic (see results). We describe these continuous distributions in detail and report descriptive statistics by intervention arm and assessment period.
When planning this pilot study we anticipated floor effects. Specifically, we expected a relatively high proportion of participants in both study arms would have substantial improvements with respect to opioid outcomes as a result of initiating buprenorphine treatment. Thus, a priori we determined that a 10% difference in opioid positive outcomes at follow-up would represent a clinically meaningful improvement.
3. RESULTS
Participants averaged 41.1 (± 11.3) years of age, 32 (65.3%) were male, and 42 (85.7%) were non-Latino White (Table 1). Fourteen (28.6%) reported they had ever received prescribed buprenorphine. The mean rate of illicit opioid use in the 30 days prior to baseline was 25.2 (± 7.1) days. Background characteristics were similar across groups although the HE arm tended to have a higher percentage of non-Latino Whites (92.0%) than the DT arm (79.2%).
Table 1.
Participant Characteristics by Intervention Arm.
| Cohort (n=49) |
HE (n=25) |
DT (n=24) |
Diff (95% CI)a | |
|---|---|---|---|---|
| Age | 41.1 (± 11.3) | 40.6 (± 12.0) | 41.7 (± 10.8) | −1.1 (−7.6; 5.5) |
| Gender (Male) | 32 (65.3%) | 16 (64.0%) | 16 (66.7%) | 2.7 (−29.3; 24.0) |
| Non-Latino White (Yes) | 42 (85.7%) | 23 (92.0%) | 19 (79.2%) | 12.8 (−6.5; 32.3) |
| Ever Prescribed Buprenorphine (Yes) |
14 (28.6%) | 6 (24.0%) | 8 (33.3%) | −9.3 (−34.6; 15.9) |
| Days / 30 Non RX Opioid Useb | 25.2 (± 7.1) | 24.6 (± 8.7) | 26.0 (± 5.0) | −1.4 (−5.5; 2.7) |
| Dropped Out of Treatment (Yes) | 12 (24.0%) | 6 (24.0%) | 6 (25.0%) | −1.0 (−25.1; 23.1) |
| Opioid Abstinent at 3 months (Yes) |
10 (20.4%) | 4 (16.0%) | 6 (25.0%) | 9.0 (−13.5; 31.5) |
Between group difference in mean or % and 95% confidence interval estimate.
Days / 30 days participants reported using opioids on the baseline time-line follow-back.
A total of 12 participants (24.5%) dropped out of treatment; rates of dropout were 24% and 25% in the HE and DT arms, respectively (Table 1). Five subjects (2 randomized to HE and 3 randomized to DT) had dropped out of the treatment protocol by week 1, 5 additional subjects (2 randomized to HE and 3 randomized to DT) had dropped out prior to the 1-month follow-up, and 2 more (both in the HE arm) dropped out prior to receiving their last buprenorphine prescription. Ten subjects were opioid abstinent (defined as no positive or missed toxicology tests at months 1-, 2-, or 3-months, and no self-reported illicit opioid use on the TLFB during the 3-month follow-up). Six (25.0%) participants randomized to DT and 4 (16.0%) of participants randomized to HE were opioid abstinent.
Concordance between self-reported opioid use and urine toxicology results during follow-up was good. Of 52 positive toxicology tests, only 1 subject failed to self-report illicit opioid use during the corresponding assessment period. Participants self-reported opioid use on 18 occasions which were not confirmed by toxicology screens; this was not unexpected given the 30-day self-reporting period and the shorter time frame for opioid use captured by toxicologic testing.
Table 2 gives between group differences with respect to any opioid use a 1-, 2-, and 3-month assessments. The intent-to-treat analysis assumes all persons who dropped out of treatment were using opioids. Persons randomized to DT had lower rates of opioid use at all three follow-ups, though between group differences were substantively small. At 2-months the difference was 56.0% (HE) compared to 50.0% (DT), and at 3-months 72% of persons randomized to HE were opioid positive compared with 62.5% of persons randomized to DT. The as-treated analysis gives results that are generally consistent with the intent-to-treat analysis (Table 2). The estimated population-averaged OR, adjusted for month and baseline frequency of opioid use, was 0.68 (95%CI 0.25; 1.84) for the intent-to-treat analysis and 0.59 (0.20; 1.68) for the as-treated analysis.
Table 2.
Any Opioid Usea by Intervention Group and Month of Follow-Up.
| INTENT TO TREATb | AS TREATEDc | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Opioid + at | HE (n = 25) |
DT (n = 24) |
Diff (95% CI)d | HE (n = e) |
DT (n = f) |
Diff (95% CI)c |
| Month 1 | 15 (60.0%) | 14 (58.3%) | 1.7 (−25.9; 29.2) | 11 (52.4%) | 8 (44.4%) | 7.9 (−23.4; 39.3) |
| Month 2 | 14 (56.0%) | 12 (50.0%) | 6.0 (−21.9; 33.9) | 8 (42.1%) | 6 (33.3%) | 8.8 (−22.3; 39.9) |
| Month 3 | 18 (72.0%) | 15 (62.5%) | 9.5 (−16.7; 35.7) | 10 (58.8%) | 9 (50.0%) | 8.8 (−24.1; 41.7) |
Any self-reported opioid use or positive urine toxicology test at 1-, 2, or 3-month assessments.
Assumes dropouts were opioid positive.
Observed data from participants in the study protocol.
Between group difference in % testing opioid+ and 95% confidence interval estimate for the between group difference.
The number of available observations were 21, 19, and 17 at 1-, 2-, and 3-month assessments, respectively.
The number of available observations was 18 at all follow-ups..
In Table 3 we give the categorical distribution of self-reported opioid use frequency by intervention arm and follow-up assessment. As expected, in both study arms, mean and median frequency of self-reported illicit opioid use was much lower during follow-up than at baseline. The distributions are extremely skewed with the modal response category at the lower limit of 0. Most participants who report any use report using illicit opioids on only 1-2 days during any assessment period. At all 3-time points, between group differences in mean frequency of use are lower in DT arm than in the HE arm.
Table 3.
Distribution of Self-Reported Illicit Opioid Use by Intervention Group and Month of Follow-Up.
| MONTH 1 | MONTH 2 | MONTH 3 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| DAYS USED |
HE (n = 21) |
DT (n = 18) |
HE (n = 19) |
DT (n = 18) |
HE (n = 17) |
DT (n = 18) |
| None | 10 (47.6%) |
11 (61.1%) |
12 (63.2%) |
14 (77.8%) |
10 (58.8%) |
10 (55.6%) |
| 1-2 Days | 6 (28.6%) | 6 (33.3%) | 5 (26.3%) | 4 (22.2%) | 5 (29.4%) | 7 (38.9%) |
| 3-5 Days | 4 (19.0%) | 1 (5.6%) | 1 (4.3%) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) |
| 6-10 Days | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 11-20 Days | 1 (4.8%) | 0 (0.0%) | 1 (5.3%) | 0 (0.0%) | 1 (5.9%) | 0 (0.0%) |
| > 20 Days | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.9%) | 0 (0.0%) |
|
| ||||||
|
Mean (±
SD) |
1.67 | 0.67 | 1.68 | 0.39 | 2.29 | 0.72 |
| SD | 2.83 | 1.24 | 4.57 | 0.78 | 5.25 | 1.07 |
| Median | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
4. DISCUSSION
We tested the preliminary efficacy of a distress tolerance intervention to reduce ongoing illicit opioid use among person initiating buprenorphine treatment and found a small improvement in opioid use during the first three months of treatment compared to a health education control condition. This DT treatment incorporates elements from exposure-based and acceptance-based treatment approaches and participants demonstrated good treatment attendance by associating the behavioral intervention sessions with buprenorphine dosing visits.
Accumulating evidence indicates that ACT is an efficacious treatment approach across a variety of clinical problem areas, such as affective disorders (Zettle, 1984; Zettle and Hayes, 1986; Zettle and Rains, 1989) and anxiety disorders, including obsessive-compulsive disorders, agoraphobia (Hayes, 1987; Hayes et al., 1990), stress in the workplace (Bond and Bunce, 2000), and social anxiety (Block, 2002). Although a number of studies have shown promising results for smoking cessation (Bricker et al., 2010; Brown et al., 2008, 2011, 2013; Gifford et al., 2004; Hernandez-Lopez et al., 2009), very limited research has examined the use of ACT for the treatment of other substance use disorders such as opioid dependence (Hayes et al., 2004; Stotts et al., 2012, 2009). We chose to focus on buprenorphine initiators because this growing population is at high risk for treatment drop-out and drug relapse, and seeks addiction treatment in primary care settings, which are increasingly involving behavioral health specialists and counselors (Butler et al., 2008), and offer the opportunity to begin a behavioral intervention prior to an induction date. Given that inability or reduced ability to tolerate distress interferes with efforts to establish longer-term opioid-free behavior change (Strong et al., 2012), we hypothesized that persons initiating buprenorphine treatment might benefit from learning new skills or strategies to tolerate the discomfort of withdrawal symptoms, cravings, and negative affect during early abstinence.
Although the rate of illicit opioid use was lower at the 1, 2 and 3 month assessments, there was no difference by treatment assignment in persons leaving treatment, a signal of relapse to drug use. The DT treatment had a modest positive effect on illicit opioid use. Readers should note that sample size was small and 95% confidence interval estimates were large and failed to exclude 0. We offer several explanations for this result. First, much of the distress of withdrawal, necessary at the time of buprenorphine initiation but transient, was ameliorated by the medication itself. Opioid replacement may have lessened the effect of a behavioral intervention; we chose a buprenorphine dose (16 mg) associated with rates of retention higher than rates seen in studies of 8 mg (Mattick et al., 2008). Still, fixed buprenorphine dosing is a study limitation; symptom-based dosing may lead to higher average daily doses greater than 16mg and perhaps better outcomes (Weiss et al., 2014). Over one-third of participants were fully abstinent over the 3-month follow-up; most others reported only infrequent used of illicit opioids (to be positive at the monthly assessment a participant had to report any use during the month or have a positive toxicology). In addition, persons assigned to HE received the structure associated with multiple medical visits and counseling sessions, which may have further mitigated finding strong effects of the DT treatment. Second, the intervention, based in metaphor, was linguistically abstract, and we believe this intervention could improve with the use of more concrete ways to use acceptance-based techniques. .Still, the low level of illicit opioid use through all three months of treatment suggests the power of external triggers. As devised, our DT intervention may not have provided tools to develop alternative strategies to combat these triggers. We interpret the lack of statistically significant differences in our primary outcomes cautiously as our study was likely underpowered (Kraemer et al., 2006). Our focus instead, was on testing preliminary efficacy of DT and to determine if a larger-scale, fully powered trial, would be worthwhile in this population.
We achieved approximately a 10% difference in opioid use by the three-month assessment, the a priori target for meaningful group difference. It remains possible that we could have seen a greater effect beyond three months when our intervention’s focus on the identification of personal values might engender a greater willingness to persist in choosing not to use opioids when provoked by craving or negative affect. Abstinence-oriented approaches for the treatment of opioid dependence that complement buprenorphine, such as our DT treatment, will be necessary to optimize outcomes. If a 10% difference in opioid use is considered to have important clinical and public health meaning, our DT intervention may be worthy of testing in a large clinical trial.
Highlights.
Distress Tolerance (DT) participants had high rates of session attendance, demonstrating feasibility
DT participants had lower rates of opioid use at all follow-up assessments vs health education (HE)
Rates of buprenorphine dropout were 24% and 25% in the HE and DT arms, respectively
Figure 1.
Participant Flow Diagram
Acknowledgments
Role of Funding Source
This study was funded by the National Institute on Drug Abuse (R34 DA032767). Dr. Stein is a recipient of a NIDA Mid-Career Investigator Award (K24 DA00512). NIDA had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Author Stein: Funded as PI of this award, Dr. Stein designed and implemented the study protocol, reviewed all data analysis and wrote the first draft of paper.
Author Herman: Served as Project Manager for this award, supervised all data collection and entry, assisted with treatment fidelity monitoring, reviewed and edited the manuscript.
Author Moitra: Served as study interventionist, reviewed and edited the manuscript.
Author Hecht: Helped develop the treatment manual, served as study interventionist, reviewed and edited the manuscript.
Author Lopez: Served as principal research staff, organizing all participant activities including assessments and follow-ups, also reviewed and edited the manuscript.
Author Anderson: Served as data analyst and statistician for this project, reviewed and edited the manuscript.
Author Brown: Worked with Dr. Stein in treatment development and manual creation, assisted with treatment fidelity monitoring, reviewed and edited the manuscript.
Author Disclosures
Conflict of Interest
No Conflict Declared
REFERENCES
- Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst. Rev. 2011:CD004147. doi: 10.1002/14651858.CD004147.pub4. doi: 10.1002/14651858.CD004147.pub4. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic And Statistical Manual Of Mental Disorders. 4th Ed. APA; Washington, DC: 1994. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol. Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Block JA. Unpublished doctoral dissertation. University at Albany, State University of New York; Albany, NY: 2002. Acceptance Or Change Of Private Experiences: A Comparative Analysis In College Students With Public Speaking Anxiety. [Google Scholar]
- Bond FW, Bunce D. Mediators of change in emotion-focused and problem-focused worksite stress management interventions. J. Occup. Health Psychol. 2000;5:156–163. doi: 10.1037//1076-8998.5.1.156. [DOI] [PubMed] [Google Scholar]
- Boothby LA, Doering PL. Buprenorphine for the treatment of opioid dependence. Am. J. Health. Syst. Pharm. 2007;64:266–272. doi: 10.2146/ajhp060403. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. J. Abnorm. Psychol. 2003;112:448–456. doi: 10.1037/0021-843x.112.3.448. [DOI] [PubMed] [Google Scholar]
- Bricker JB, Mann SL, Marek PM, Liu J, Peterson AV. Telephone-delivered Acceptance and Commitment Therapy for adult smoking cessation: a feasibility study. Nicotine Tob. Res. 2010;12:454–458. doi: 10.1093/ntr/ntq002. doi: 10.1093/ntr/ntq002. [DOI] [PubMed] [Google Scholar]
- Brown RA, Bloom EL, Hecht J, Moitra E, Herman DS, Stein MD. A pilot study of a distress tolerance treatment for opiate-dependent patients initiating buprenorphine: rationale, methodology, and outcomes. Behav. Modif. 2014;38:730–759. doi: 10.1177/0145445514538279. doi: 10.1177/0145445514538279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. J. Abnorm. Psychol. 2002;111:180–185. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, Niaura R, Price LH. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine Tob. Res. 2009;11:493–502. doi: 10.1093/ntr/ntp041. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Hayes SC, Wilson KG, Gifford EV. Distress tolerance treatment for early-lapse smokers: rationale, program description, and preliminary findings. Behav. Modif. 2008;32:302–332. doi: 10.1177/0145445507309024. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, Lejuez CW, Zvolensky M, Gifford EV, Hayes SC. Efficacy Of Distress Tolerance/ACT Treatment Vs. Standard Behavioral Treatment For Early Lapse Smokers; Annual Meeting of the Society for Behavioral Medicine; Washington, DC. 2011. [Google Scholar]
- Brown RA, Reed KM, Bloom EL, Minami H, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Gifford EV, Hayes SC. Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine Tob. Res. 2013;15:2005–2015. doi: 10.1093/ntr/ntt093. doi: 10.1093/ntr/ntt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M, Kane RL, McAlpine D, Kathol RG, Fu SS, Hagedorn H, Wilt TJ. Integration of mental health/substance abuse and primary care. Evid. Rep. Technol. Assess. (Full Rep.) 2008;173:1–362. [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Giovanniello A, Sacajiu G, Whitley S, Mund P, Beil R, Sohler N. Buprenorphine treatment in an urban community health center: what to expect. Fam. Med. 2008;40:500–506. [PMC free article] [PubMed] [Google Scholar]
- Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (PASAT): norms for age, education, and ethnicity. Assessment. 1998;5:375–387. doi: 10.1177/107319119800500407. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J. Consult. Clin. Psychol. 2000;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Barry DT, Sullivan LE, Cutter CJ, Moore BA, O’Connor PG, Schottenfeld RS. A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine. Am. J. Med. 2013;126:74 e11–77. doi: 10.1016/j.amjmed.2012.07.005. doi: 10.1016/j.amjmed.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O’Connor PG, Schottenfeld RS. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N. Engl. J. Med. 2006;355:365–374. doi: 10.1056/NEJMoa055255. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Pantalon MV, Pakes JP, O’Connor PG, Chawarski M, Schottenfeld RS. Treatment of heroin dependence with buprenorphine in primary care. Am. J. Drug Alcohol Abuse. 2002;28:231–241. doi: 10.1081/ada-120002972. [DOI] [PubMed] [Google Scholar]
- Finch JW, Kamien JB, Amass L. Two-year experience with buprenorphine-naloxone (suboxone) for maintenance treatment of opioid dependence within a private practice setting. J. Addict. Med. 2007;1:104–110. doi: 10.1097/ADM.0b013e31809b5df2. doi: doi: 10.1097/ADM.0b013e31809b5df2. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, Collins J, Raisch D, Casadonte P, Goldsmith RJ, Ling W, Malkemeker U, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N. Engl. J. Med. 2003;349:949–958. doi: 10.1056/NEJMoa022164. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- Gifford EV. Setting a course for behavior change: the verbal context of acceptance. In: Hayes SC, Jacobson NS, Follette VM, Dougher MJ, editors. Acceptance And Change: Content And Context In Psychotherapy. Context Press; Reno, NV: 1994. pp. 218–222. [Google Scholar]
- Gifford EV, Hayes SC. Discrimination Training And The Function Of Acceptance; Paper presented at the Paper presented at the meeting of the Association for Behavior Analysis; Chicago, IL. 1997. [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, Rasmussen-Hall ML, Palm KM. Acceptance-based treatment for smoking cessation. Behav. Ther. 2004;35:689–706. [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczynski J, Mitchell SG, Jaffe JH, O’Grady KE, Olsen YK, Schwartz RP. Leaving buprenorphine treatment: patients’ reasons for cessation of care. J. Subst. Abuse Treat. 2014;46:356–361. doi: 10.1016/j.jsat.2013.10.004. doi: 10.1016/j.jsat.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JW, Hilbe JM. Generalized Estimating Equations. Chapman and Hall/CRC; Boca Raton, FL: 2002. [Google Scholar]
- Hayes S, Wilson K, Gifford E, Bissett R, Piasecki M, Batten S, Byrd M, Gregg J. A preliminary trial of twelve-step facilitation and acceptance and commitment therapy with polysubstance-abusing methadone-maintained opiate addicts. Behav. Ther. 2004;35:667–688. [Google Scholar]
- Hayes SC. A contextual approach to therapeutic change. In: Jacobson N, editor. Psychotherapists In Clinical Practice. Guilford Press; New York: 1987. pp. 327–387. [Google Scholar]
- Hayes SC. The Acceptance and Action Questionnaire - II. 2006. Unpublished manuscript. [Google Scholar]
- Hayes SC, Afari N, McCurry SM, Wilson KG. The Efficacy Of Comprehensive Distancing In The Treatment Of Agoraphobia; Paper presented at the Paper presented at the annual meeting of the Association for Behavior Analysis; Nashville, TN. 1990. [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy: An Experiential Approach To Behavior Change. The Guilford Press; New York: 1999. [Google Scholar]
- Hendershot CS, Witkiewitz K, George WH, Marlatt GA. Relapse prevention for addictive behaviors. Subst. Abuse Treat. Prev. Policy. 2011;6:17. doi: 10.1186/1747-597X-6-17. doi: 10.1186/1747-597X-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez M, Luciano MC, Bricker JB, Roales-Nieto JG, Montesinos F. Acceptance and commitment therapy for smoking cessation: a preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychol. Addict. Behav. 2009;23:723–730. doi: 10.1037/a0017632. doi: 10.1037/a0017632. [DOI] [PubMed] [Google Scholar]
- Holdwick DJ, Jr., Wingenfeld SA. The subjective experience of PASAT testing. Does the PASAT induce negative mood? Arch. Clin. Neuropsychol. 1999;14:273–284. doi: 10.1093/arclin/14.3.273. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong KI, Doebrick C, Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp. Clin. Psychopharmacol. 2007;15:134–143. doi: 10.1037/1064-1297.15.2.134. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch. Gen. Psychiatry. 2006;63:484–489. doi: 10.1001/archpsyc.63.5.484. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- Lee JD, Grossman E, DiRocco D, Gourevitch MN. Home buprenorphine/naloxone induction in primary care. J. Gen. Intern. Med. 2009;24:226–232. doi: 10.1007/s11606-008-0866-8. doi: 10.1007/s11606-008-0866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Paulson A, Daughters SB, Bornovalova MA, Zvolensky MJ. The association between heroin use and anxiety sensitivity among innercity individuals in residential drug use treatment. Behav. Res. Ther. 2006;44:667–677. doi: 10.1016/j.brat.2005.04.006. doi: 10.1016/j.brat.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Ang A, Jenkins J, Fahey J. Comparison of behavioral treatment conditions in buprenorphine maintenance. Addiction. 2013;108:1788–1798. doi: 10.1111/add.12266. doi: 10.1111/add.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Pantelis C. Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction. 2004;99:1491–1502. doi: 10.1111/j.1360-0443.2004.00808.x. doi: 10.1111/j.1360-0443.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- Luoma JB, Hayes SC, Walser R. Learning Acceptance And Commitment Therapy: A Skills Training Manual For Therapists. New Harbinger Press; Oakland, CA: 2007. [Google Scholar]
- Magill M, Ray LA. Cognitive-behavioral treatment with adult alcohol and illicit drug users: a meta-analysis of randomized controlled trials. J. Stud. Alcohol Drugs. 2009;70:516–527. doi: 10.15288/jsad.2009.70.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Lee SJ, Salsitz EA, Kolodny A, Whitley SD, Taubes T, Seewald R, Joseph H, Kayman DJ, Fong C, Marsch LA, Rosenblum A. Outcomes of buprenorphine maintenance in office-based practice. J. Addict. Dis. 2007;26:13–23. doi: 10.1300/J069v26n02_03. doi: 10.1300/J069v26n02_03. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2008:CD002207. doi: 10.1002/14651858.CD002207.pub3. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Ann. Fam. Med. 2007;5:146–150. doi: 10.1370/afm.665. doi: 5/2/146 [pii] 10.1370/afm.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn EP, Brandon TH, Copeland AL. Is task persistence related to smoking and substance abuse? The application of learned industriousness theory to addictive behaviors. Exp. Clin. Psychopharmacol. 1996;4:186–190. [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. doi: 10.1080/09652140020016996. [DOI] [PubMed] [Google Scholar]
- Soeffing JM, Martin LD, Fingerhood MI, Jasinski DR, Rastegar DA. Buprenorphine maintenance treatment in a primary care setting: outcomes at 1 year. J. Subst. Abuse Treat. 2009;37:426–430. doi: 10.1016/j.jsat.2009.05.003. doi: 10.1016/j.jsat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. J. Gen. Intern. Med. 2005;20:1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Herman DS, Kettavong M, Cioe PA, Friedmann PD, Tellioglu T, Anderson BJ. Antidepressant treatment does not improve buprenorphine retention among opioid-dependent persons. J. Subst. Abuse Treat. 2010;39:157–166. doi: 10.1016/j.jsat.2010.05.014. doi: 10.1016/j.jsat.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotts AL, Green C, Masuda A, Grabowski J, Wilson K, Northrup TF, Moeller FG, Schmitz JM. A stage I pilot study of acceptance and commitment therapy for methadone detoxification. Drug Alcohol Depend. 2012;125:215–222. doi: 10.1016/j.drugalcdep.2012.02.015. doi: 10.1016/j.drugalcdep.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotts AL, Masuda A, Wilson K. Using acceptance and commitment therapy during methadone dose reduction: rationale, treatment description, and a case report. Cogn. Behav. Pract. 2009;16:205–213. doi: 10.1016/j.cbpra.2008.08.003. doi: 10.1016/j.cbpra.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DR, Brown RA, Sims M, Herman DS, Anderson BJ, Stein MD. Persistence on a stress-challenge task before initiating buprenorphine treatment was associated with successful transition from opioid use to early abstinence. J. Addict. Med. 2012;6:219–225. doi: 10.1097/ADM.0b013e31825d927f. doi: 10.1097/ADM.0b013e31825d927f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2012. [Google Scholar]
- Tsui JI, Anderson BJ, Strong DR, Stein MD. Craving predicts opioid use in opioid-dependent patients initiating buprenorphine treatment: a longitudinal study. Am. J. Drug Alcohol Abuse. 2014;40:163–169. doi: 10.3109/00952990.2013.848875. doi: 10.3109/00952990.2013.848875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tull MT, Schulzinger D, Schmidt NB, Zvolensky MJ, Lejuez CW. Development and initial examination of a brief intervention for heightened anxiety sensitivity among heroin users. Behav. Modif. 2007;31:220–242. doi: 10.1177/0145445506297020. doi: 31/2/220 [pii] 10.1177/0145445506297020. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Potter JS, Dodd DR, Dreifuss JA, Connery HS, Carroll KM. Who benefits from additional drug counseling among prescription opioid-dependent patients receiving buprenorphine-naloxone and standard medical management? Drug Alcohol Depend. 2014;140:118–122. doi: 10.1016/j.drugalcdep.2014.04.005. doi: 10.1016/j.drugalcdep.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J. Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am. Psychol. 2004;59:224–235. doi: 10.1037/0003-066X.59.4.224. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Zettle RD. Unpublished doctoral dissertion. University of North Carolina; Greensboro, NC: 1984. Cognitive therapy of depression: a conceptual and empirical analysis of component and process issues. [Google Scholar]
- Zettle RD, Hayes SC. Dysfunctional control by client verbal behavior: the context of reason-giving. Anal. Verbal. Behav. 1986;4:30–38. doi: 10.1007/BF03392813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettle RD, Rains JC. Group cognitive and contextual therapies in treatment of depression. J. Clin. Psychol. 1989;45:436–445. doi: 10.1002/1097-4679(198905)45:3<436::aid-jclp2270450314>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]