Abstract
Background
Wound healing is an interaction of a complex signaling cascade of cellular events, including inflammation, proliferation, and maturation. K+ channels modulate the mitogen-activated protein kinase (MAPK) signaling pathway. Here, we investigated whether K+ channel-activated MAPK signaling directs collagen synthesis and angiogenesis in wound healing.
Methods
The human skin fibroblast HS27 cell line was used to examine cell viability and collagen synthesis after potassium chloride (KCl) treatment by Cell Counting Kit-8 (CCK-8) and western blotting. To investigate whether K+ ion channels function upstream of MAPK signaling, thus affecting collagen synthesis and angiogenesis, we examined alteration of MAPK expression after treatment with KCl (channel inhibitor), NS1619 (channel activator), or kinase inhibitors. To research the effect of KCl on angiogenesis, angiogenesis-related proteins such as thrombospondin 1 (TSP1), anti-angiogenic factor, basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), pro-angiogenic factor were assayed by western blot.
Results
The viability of HS27 cells was not affected by 25 mM KCl. Collagen synthesis increased dependent on time and concentration of KCl exposure. The phosphorylations of MAPK proteins such as extracellular-signal-regulated kinase (ERK) and p38 increased about 2.5-3 fold in the KCl treatment cells and were inhibited by treatment of NS1619. TSP1 expression increased by 100%, bFGF expression decreased by 40%, and there is no significant differences in the VEGF level by KCl treatment, TSP1 was inhibited by NS1619 or kinase inhibitors.
Conclusions
Our results suggest that KCl may function as a therapeutic agent for wound healing in the skin through MAPK signaling mediated by the K+ ion channel.
Keywords: Potassium channels, Mitogen activated protein kinases, Wound healing, Angiogenesis
INTRODUCTION
When tissue is disrupted in vertebrates, the wound healing process begins, which is generally divided into three phases: inflammation, proliferation, and maturation [1]. Each phase is regulated by cytokines, growth factors, and their cellular receptors [2]. In this study, we focused on 1) collagen synthesis and 2) erythema caused by angiogenesis during wound healing.
First, collagen is a main protein of human skin, allowing for much of the skin's remodeling and turnover [3]. Skin fibroblasts generate a precursor molecule called procollagen, which is converted into collagen [4,5]. Previously, collagen was thought to act only as a structural support. However, it has been reported that collagen is a major factor of wound healing. There are two important regulators of collagen production: transforming growth factor beta (TGFβ) and activator protein-1 [6]. TGFβ is a multifunctional cytokine and improves wound healing by promoting fibroblast growth [7,8] and collagen I and III formation [9] and by preventing matrix degradation through induction of protease inhibitors. There is a close correlation between the TGFβR-mitogen-activated protein kinases (MAPKs) [10,11]. The MAP kinases are grouped into three families, which are extracellular-signal-regulated kinases (ERKs), Jun amino-terminal kinases (JNKs), and stress-activated protein kinases (p38/SAPKs). Each cascade is initiated by specific extracellular and intracellular stimuli such as several stresses, growth factors, cytokines, hormones, oxidative stress and mitogens. It leads to activation of specific MAPK following activation of MAPK kinase kinase and MAPK kinase independently or dependently of each other. Activated MAPK phosphorylates several substrates in the cytosol and nucleus to occur about changes in protein function and gene expression that carry out the biological response including cell proliferation, differentiation, inflammation, and cell death in eukaryotes. And dysregulation of MAPK pathways has been implicated in many diseases.
Second, erythema is an abnormal skin condition characterized by redness following stresses, including exposure to heat, infections, allergens, and radiation (sunlight, ultraviolet, X-ray). Erythema is continued by late stage of wound healing after injury. Erythema is a rapid angiogenic response during wound healing in skin. Angiogenesis is a critical factor of the normal healing process. New blood vessels deliver oxygen, nutrients, and growth factors to injured tissues. On the other hand, erythema is a common side effect following treatment therapy such as laser procedures on skin [12]. In these reason, postoperative cares, including cooling sprays, light-based modalities and dressings are conducted for a relief of erythema. Therefore, it is necessary to study an adjustable treatment for relief of erythema by modulation of thrombospondin 1 (TSP1) level, but dose not delay early stage of wound healing. TSP1 is major protein during angiogenesis and tumorigenesis [13]. And it is a trimeric, calcium-binding molecule composed of several domains. Both positive and negative regulation of cell adhesion and growth have been influenced by to TSP1. TSP1 also have an effect on neuronal migration in the rostral migratory stream [14]. Andrikopoulos et al. [15] reported that voltage-gated ion channels may be novel targets for controlling angiogenesis [16]. Wound healing might employ several possible mechanisms via ion channel modulation, including the inhibition of several processes: MAPK-dependent activation, the reception of anti-angiogenic TSP1, vascular endothelial growth factor (VEGF), pro-angiogenic factor and VEGF receptor expression, and matrix metalloproteinase inhibitor expression. However, the direct mechanism of wound healing by KCl-mediated ion channel function in skin remains unclear. Therefore, we showed that ion channel regulation, mediated by KCl, functions in wound healing as well as relief of erythema by regulation of TSP1 level.
METHODS
Cell culture
HS27 cells were grown to confluence in high-glucose dulbecco modified eagle medium with 10% fetal bovine serum supplemented with penicillin (100 units/mL) and streptomycin (100 mg/mL). Cells were cultured at 37℃ in a humidified atmosphere of 5% CO2.
Western blot
Cultured cells was rinsed with phosphate-buffered saline and incubated for 20 minutes in lysis buffer with 1 mM phenylmethanesulfonyl fluoride. After centrifugation at 14,000 rpm for 10 minutes, protein concentration was quantified by the Bradford protein assay. For immunoblotting, equal amounts of proteins were electrophoresed in 12% sodium dodecyl sulfate-polyacrylamide gel and transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA) using a transfer apparatus (Bio-Rad). The membranes were blocked for 1 hour with 5% dry milk or bovine serum albumin before addition of the primary antibody, which was incubated overnight at 4℃. Chemiluminescence of immunoreactive bands was detected with the ECL Plus Kit (GE Healthcare, Buckinghamshire, UK). Densitometric quantification of bands was performed using Multi Gauge ver. 2.3 Software (Fuji Film, Tokyo, Japan). The antibodies for procollagen type-I, VEGF, basic fibroblast growth factor (bFGF), and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The MAPK Family Antibody Sampler Kit (measuring expression of the total- and phospho-MAPK proteins). The antibodies for TGFβ and TSP1 and anti-mouse, anti-rabbit, and anti-goat HRP-conjugated secondary antibodies were purchased from Abcam (Cambridge, MA, USA). The relative expression of each protein was quantified by densitometric measurement after western blotting with the Multi Gauge ver. 3.1 Software. All data were obtained from triplicate experiments.
Cell viability
To examine cell viability, HS27 cells were plated 24 hours before the experiment in 12-well plates at a density of 3×104 cells/well. The cells were then treated for 24 hours with KCl at several concentrations (0, 4, 8, 12, and 25 mM). Then, cells were incubated in growth medium with Cell Counting Kit-8 (CCK-8) Reagent (Dojindo, Tabaru, Japan). After incubation for 2 hours, absorbance of each sample was measured at 450 nm using spectrophotometer.
Chemicals
KCl was dissolved in water to prepare a stock 2.5 M solution for subsequent dilution. NS1619 (Sigma, St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) to prepare a stock 10 mM solution. Each chemical was added to the experimental solutions, and the final concentration of DMSO never exceeded 0.1%.
Statistics
All results are expressed as the means±standard error of at least three independent experiments. Statistical analyses were performed using Sigmaplot, and significant differences were assessed using the Student's t-test. When necessary, multiple comparisons were made using the Kruskal-Wallis test. (*P<0.05, **P<0.01, and ***P<0.001). NS indicates not significant.
RESULTS
Treatment with KCl induced collagen formation in human fibroblasts
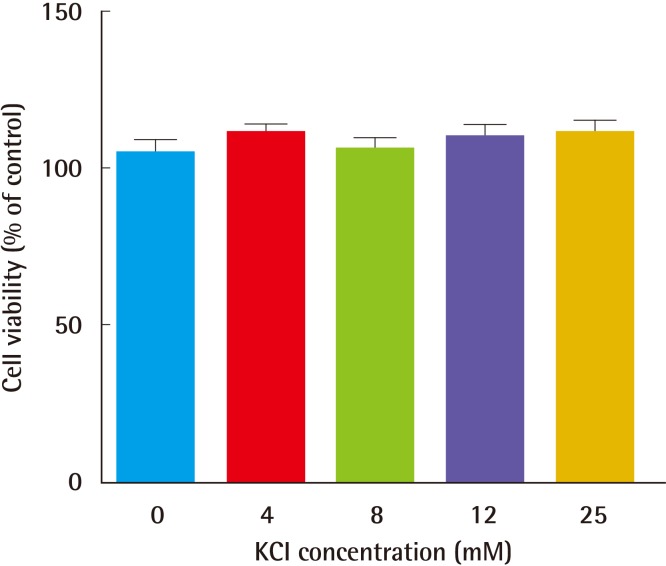
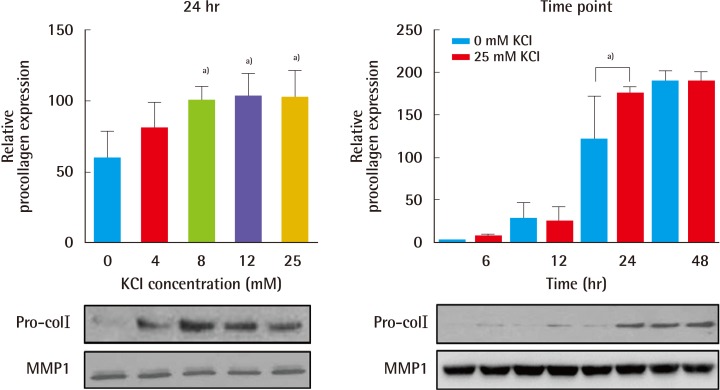
First, to test whether KCl promotes collagen formation, an appropriate KCl treatment concentration was determined based on a toxicity test. Cells were treated for 24 hours with KCl at each concentration, and cell viability was measured by a CCK analysis. Cells showed similar susceptibility to each KCl concentration (Fig. 1). Therefore, we used 25 mM KCl for further experimentation, because this concentration of KCl did not affect cell death or proliferation. In addition, expression of procollagen type-I was examined after KCl treatment. To compare collagen expression in KCl-treated cells with that in controls, we measured procollagen levels in HS27 cells. KCl led to a concentration- and time-dependent increase of procollagen type-I levels without cell damage (Fig. 2).
Fig. 1.
Cell viability with KCl treatment
Cells were treated with KCl at each concentration for 24 hours, and cell viability was measured by a CCK analysis. Cells showed similar susceptibility to KCl treatment. KCI, potassium chloride; CCK, Cell Counting Kit.
Fig. 2.
KCl treatment increased procollagen type-I levels
Formation of procollagen type-I (Pro-colI) was examined after KCl treatment. Procollagen protein levels increased the most with 25 mM KCl treatment for 24 hours. MMP1 was used for loading control (a)P<0.05). KCI, potassium chloride; MMP-1, matrix metalloproteinase 1.
KCl-induced collagen formation is mediated by MAPK pathway in human fibroblast
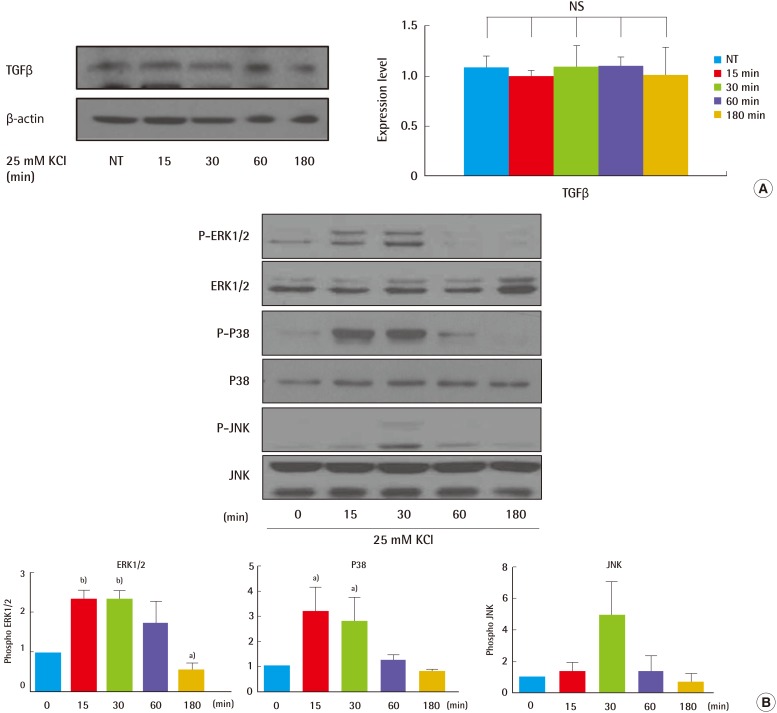
To analyze whether KCl may modulate TGFβ signaling related to collagen synthesis in skin, HS27 cells were treated with 25 mM KCl for different durations. We performed a comparative immunoblot analysis to investigate expression of MAPK proteins, including ERK and p38. Phosphorylated ERK and p38 levels increased by 2.5- to 3-fold in the KCl-treated cells relative to non-treated cells, at the 15 minutes and 30 minutes time periods (Fig. 3B). However, expression of TGFβ an upstream regulator of the MAPK signaling pathway, was not altered by KCl treatment at any time point (Fig. 3A). Collectively, these data indicate that KCl-induced collagen synthesis is solely mediated via the MAPK pathway, independent of the TGFβR.
Fig. 3.
KCl treatment altered TGFβ signaling in cells
Activation of TGFβ signaling was examined after KCl treatment. HS27 cells were treated with 25 mM KCl for 0, 15, 30, 60, and 180 minutes. (A) Expression of TGFβ was not altered during KCl incubation. (B) Levels of phosphorylated MAPK proteins, such as ERK1/2 and p38, were highest at 30 minutes (a)P<0.05, b)P<0.01). TGFβ, transforming growth factor beta; KCl, potassium chloride; MAPK, mitogen-activated protein kinase; NT, not treated; NS, not significant; P-ERK, phospho-extracellular signal-regulated kinases; P-JNK, phospho-c-Jun N-terminal kinase.
KCl regulation of MAPK cascades is mediated by K+ ion channels
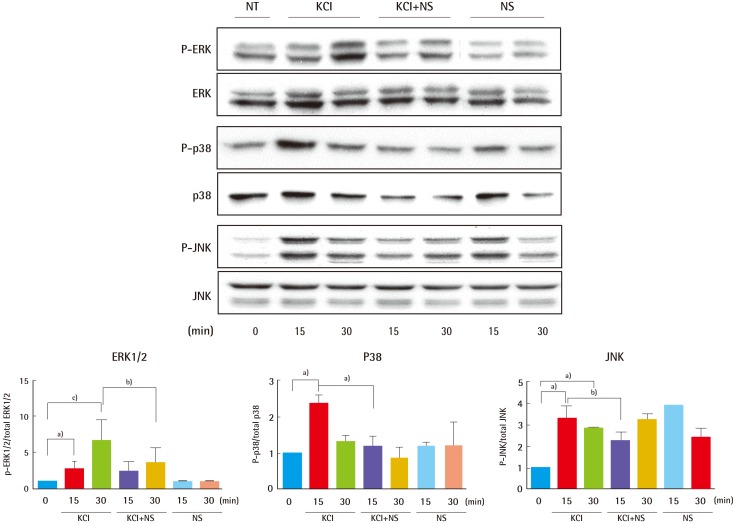
Based on the above results, KCl can activate each of the three major MAPK signaling pathways: ERK1/2, Stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK), and p38 (Fig. 3), independently of TGFβ activation during collagen formation. Next, we verified whether MAPK signaling is regulated by an ion channel or TGFβ receptor using NS1619, an ion channel activator. Cells were treated with 25 mM KCl after 10 µM NS1619 pre-treatment for 15 minutes or 30 minutes. Compared with their levels in cells treated with KCl alone, levels of phosphorylated ERK1/2 and p38 decreased to about 45% and 50% in cells incubated with KCl after NS1619 treatment for 30 minutes and 15 minutes, respectively (Fig. 4). However, levels of phosphorylated JNK decreased at only the 15-minute time point with KCl and NS1619 treatment (Fig. 4). These result show that MAPK pathways, especially those involving ERK and p38, are regulated through modulation of ion channel activity, independent of TGFβR, and that this interaction is critical to collagen synthesis.
Fig. 4.
KCl activated MAPK cascades via ion channels
To examine whether KCl-mediated TGFβ signaling is regulated through ion channels, cells were treated KCl after NS1619 (NS) pre-treatment for 15 minutes or 30 minutes. Compared with cells treated with KCl alone, cells incubated with KCl after NS1619 pre-treatment for 30 minutes and 15 minutes showed 45% and 50% decreases in levels of phosphorylated ERK1/2 and p38, respectively (a)P<0.05, b)P<0.01, and c)P<0.001). KCl, potassium chloride; MAPK, mitogen-activated protein kinase; TGFβ, transforming growth factor beta; NT, not treated; P-ERK, phospho-extracellular signal-regulated kinases; P-JNK, phospho-c-Jun N-terminal kinase.
KCl treatment increased TSP1 expression through K+ ion channel regulation
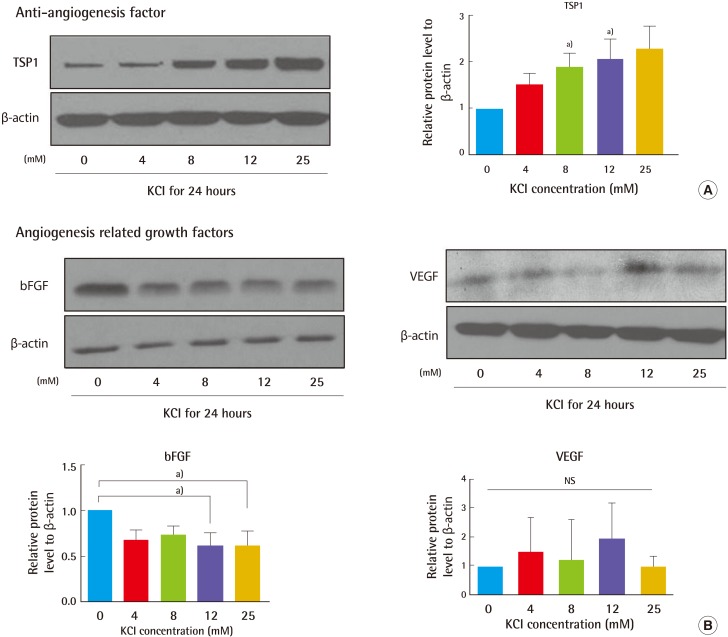
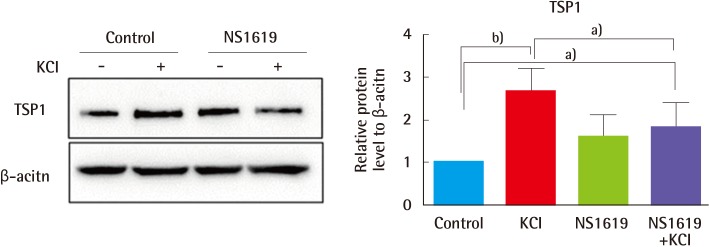
Given that collagen production is regulated by KCl-induced activation of the MAPK pathway, we investigated KCl modulation of angiogenesis related to erythema. Two different kinds of angiogenesis-related gene products, anti-angiogenic factors (TSP1) and angiogenesis factors (bFGF, VEGF), were examined. Cells were treated with KCl, and their protein expression was assayed by western blot. Of interest, 25 mM KCl-treated cells showed 100% increased TSP1 expression and 40% decreased bFGF expression (Fig. 5). No significant differences were detected in the VEGF levels. KCl-treated cells showed inhibition of angiogenesis. In order to investigate in greater detail about the relationship of TSP1 alteration between ion channels and angiogenesis with KCl treatment, cells pre-treated with NS1619 were exposed to KCl for a period of 24 hours, and TSP1 expression was determined by western blotting. Similar to our previous data, KCl treatment increased TSP1 levels by about 2.5-fold. However, KCl treatment after NS1619 pre-treatment attenuated the increased levels of TSP1 by about 30% (Fig. 6). These results show that KCl-mediated regulation of ion channels may relieve angiogenesis in wound healing.
Fig. 5.
KCl altered angiogenesis-related protein levels
(A) Expression of TSP1 in 8 and 12 mM KCl-treated cells increased by 90% and 110%, respectively, which was significantly higher than that in untreated cells. TSP1 level also increased with 25 mM KCl treatment. (B) The level of bFGF in KCl-treated cells was lower than that in non-treated cells. Proteins levels were normalized to β-actin, the protein loading control (a)P<0.05). KCI, potassium chloride; TSP1, thrombospondin 1; bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor; NS, not significant.
Fig. 6.
KCl-regulated ion channels were associated with angiogenesis
To examine whether KCl-mediated angiogenesis is regulated through ion channel activity, cells were treated with 25 mM KCl after NS1619 pre-treatment for 15 minutes. Compared with cells treated with KCl alone, cells incubated with both KCl and NS1619 showed 30% decrease in TSP1 expression. These results show that KCl-mediated ion channel regulation inhibits angiogenesis. Proteins levels were normalized to β-actin, the protein loading control (a)P<0.05 and b)P<0.01). KCI, potassium chloride; TSP1, thrombospondin 1.
Increased TSP1 levels in human skin fibroblasts are dependent on KCl-induced, ion channel-mediated MAPK activation
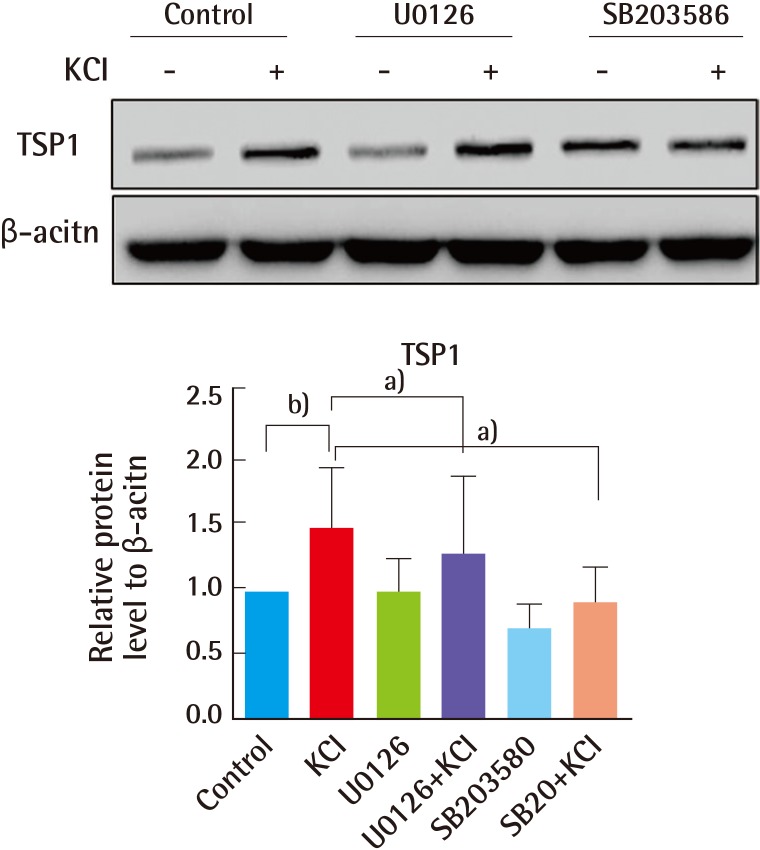
Finally, our previous data showed that MAPK signaling, mediated by ion channels, contributes to the elevated collagen production with KCl treatment. In addition, expression of TSP1 was also increased by KCl. Therefore, we wanted to further clarify whether induction of TSP1 is impacted by blocking endogenous TGFβ signaling. HS27 fibroblasts were pre-treated with inhibitors of ERK (U0126) and p38 (SB203585) for 1 hour, and then cells were incubated in KCl for 24 hours. TSP1 expression increased about 50% with KCl treatment. In addition, induction of TSP1 in U0126- and SB203585-treated cells was attenuated by 20% and 30%, respectively, relative to that in cells treated with KCl alone (Fig. 7). Based on these results, inhibition of the ion channel mediated MAPK pathway is responsible for the diminished TSP1 levels in human skin cells.
Fig. 7.
KCl and inhibitors altered TSP1 levels
TSP1 protein levels were analyzed in total lysates from cells cultured in the presence or absence of 25 mM KCl for 24 hours after pre-treatment with inhibitors of ERK [U0126], p38 [SB203585] for 1 hour. TSP1 protein levels were quantified by densitometry, and the change in TSP1 in inhibitor-treated cells was calculated. Western blotting showing that ERK and p38 inhibitors reduced TSP1 expression in KCl-treated cells. Proteins levels were normalized to β-actin, the protein loading control (a)P<0.05 and b)P<0.01). KCI, potassium chloride; TSP1, thrombospondin 1; ERK, extracellular signal-regulated kinases.
DISCUSSION
Wound healing is a natural repair response to injury and involves complex interactions between cellular and molecular cascades. In this report, we focused on collagen formation and erythema related to ion channel-mediated signal pathways. Collagen is the major component of wound healing [17,18]. For this reason, natural or synthetic collagen in sponges, membranes, and dressings is used to anchor cytoactive agents for wound healing [19]. During collagen synthesis in the proliferation phase of wound healing, various secreted cytokines and growth factors, such as TGFβ, bFGF, and platelet-derived growth factor, control the status of fibroblasts [20,21]. bFGF stimulates vascularization to increase the oxygen and nutrient supply that promotes collagen synthesis. The TGFβ signaling pathway is involved in many cellular processes, including cell growth and homeostasis, development and immune functions. TGFβ superfamily ligands phosphorylate receptor-regulated SMADs by binding to the extracellular fibroblast TGFβR [22]. Alternatively, TGFβ activates non-Smad pathways, including the three MAPK pathways: ERK, JNK, and p38 MAPK [22]. Ion channel activity modulates the MAPK pathways [23]. Investigating wounded tissue repair is necessary to understand the biological and molecular determinants of tissue regeneration and to develop new methods to promote repair.
We hypothesized that KCl has wound healing properties, which are mediated by ion channel-induced MAPK cascades, and that these pathways regulate the balance between collagen synthesis and angiogenesis. First, we investigated whether alteration in KCl levels affected cell viability, and no change was observed in the viability of 25 mM KCl-treated HS27 cells (Fig. 1). Additionally, treatment with 25 mM KCl for 24 hours resulted in a 50% increase in procollagen levels (Fig. 2), showing that treatment with 25 mM KCl specifically contributed to collagen induction observed in human fibroblasts. As a result, we hypothesized that KCl may promote wound healing by increasing collagen synthesis without cell damage in the skin. To investigate the regulatory role of KCl in collagen synthesis, mediated by MAPK, we analyzed the expression of TGFβ signaling-related proteins, such as ERK, p38, JNK, in untreated and KCl-treated cells, and we assessed how KCl treatment altered levels of signaling proteins upstream of collagen synthesis (Fig. 3). K+ channels may control epithelial repair processes [24]. Compared with cells treated with KCl alone, cells treated with KCl after NS1619 pre-treatment for 30 minutes showed 45% and 50% decreases in phosphorylated ERK1/2 and p38 (Fig. 4). Thus, KCl may promote collagen synthesis by modulation of ion channel-specific activation of MAPK pathways, independently of the TGFβR.
Angiogenesis, new blood vessel formation, is necessary for wound healing but too much angiogenesis result in erythema in impaired skin. We hypothesized that KCl may function in wound healing by regulating the balance of collagen formation and angiogenesis. TSP1 was firstly identified endogenous inhibitor of angiogenesis, and it is a multifunctional, extracellular matrix protein [15]. Further, TSP1 is critical in wound healing. TSP1 expression is typically low in normal skin, is induced acutely after wounding, and is diminished by about 14 days post-wounding [16]. Recently, it was also reported that the transient potential receptor channel 4 controls TSP1 secretion and angiogenesis in renal cell carcinoma [25]. Therefore, we investigated whether KCl regulates TSP1 expression, mediated by wound healing signaling pathways. KCl-treated cells had increased TSP1 levels, dependent on KCl concentration. bFGF protein levels were decreased in the KCl-treated cells (Fig. 5). In KCl-treated cells, the induction of TSP1 might be a negative feedback mechanism to counterbalance the effect of pro-angiogenic factors such as bFGF and VEGF.
Finally, we considered the possibility that regulation of ion channels by KCl causes upregulation of TSP1 protein, which is involved in angiogenesis and wound healing. KCl-treated cells exhibited induction of TSP1 levels, which would lead to anti-angiogenic effects. The TSP1 induction was reversed by NS-1619, a specific K+ channel opener (Fig. 6). To assess the role of this signaling pathway in the regulation of TSP1 by KCl, cells were treated with inhibitors against ERK (U0126) and p38 (SB203585). TSP1 levels were increased in KCl-treated cells, and increased TSP1 expression was downregulated after pre-treatment with ERK and p38 inhibitors (Fig. 7). Collectively, we suggest that KCl treatment may relieve angiogenesis, by inducing TSP1 expression, through ion channel-mediated ERK and p38 MAPK signaling, not TGFβ signaling.
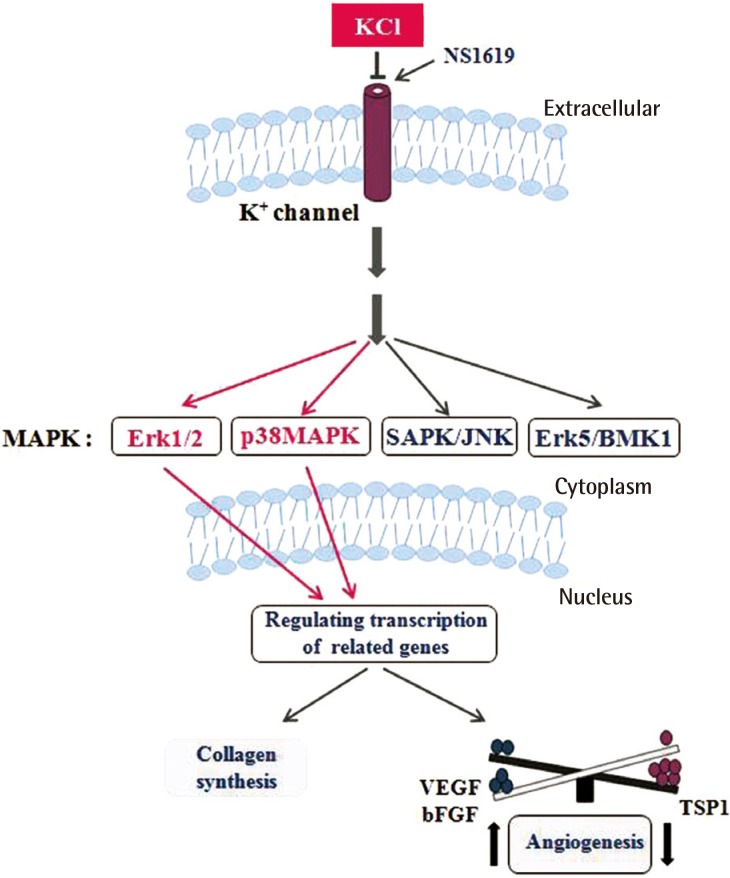
Collagen and TSP1 expression increased significantly in KCl-treated cells than in untreated cells. Collagen which is produced by KCl helps to skin's regeneration. Erythema that result by angiogenesis after wound decreases by treating KCl with induction of TSP1 level. During wound healing, the skin often shows erythema, which is caused by angiogenesis; nevertheless, angiogenesis supplies the essential elements for the remaining healing process. In addition, we established the mechanism of collagen production and inhibition of angiogenesis through the ERK and p38 pathways, which are mediated by regulation of K+ ion channels (Fig. 8), Taken together, these findings indicate that the appropriate concentration of KCl modulates the balance between anti- and pro-angiogenic mediators, leading to collagen synthesis and erythema maintaining moderate level of angiogenesis during wound healing process (Fig. 8). The results of the present study will provide important insight on the wound healing mechanism of KCl via ion channel-mediated MAPK signaling in animal model with adjust concentration of KCl. In addition, this finding may lead to the ideal application of collagen synthesis-based therapies to help modulate balance of angiogenesis in human skin.
Fig. 8.
KCl promotes wound healing through MAPK pathway
K+ channel signaling by KCl activates MAPK pathways, including Erk and p38 pathways. Activation of MAPK pathways cause increased collagen synthesis and altered expression of angiogenesis related genes. NS1619 reverse the action of KCl. KCl, potassium chloride; MAPK, mitogen-activated protein kinase; NS1619, 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazole-2-one; SAPK/JNK, stress-activated kinases/c-Jun N-terminal kinases; Erk, extracellular signal-regulated kinas; BMK1, big MAP kinase 1; VEGF, vascular endothelial growth factor; bGFG, basic fibroblast growth factor; TSP1, thrombospondin 1.
Footnotes
This study was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2010-0022472) and the Seoul National University Brain Fusion Program Research Grant.
No potential conflict of interest relevant to this article was reported.
References
- 1.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 2.Sinno H, Prakash S. Complements and the wound healing cascade: an updated review. Plast Surg Int. 2013;2013:146764. doi: 10.1155/2013/146764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oryan A. Role of collagen in soft connective tissue wound healing. Transplant Proc. 1995;27:2759–2761. [PubMed] [Google Scholar]
- 4.Bellamy G, Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc Natl Acad Sci U S A. 1971;68:1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kivirikko KI, Risteli L. Biosynthesis of collagen and its alterations in pathological states. Med Biol. 1976;54:159–186. [PubMed] [Google Scholar]
- 6.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39:258–263. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 7.Kothapalli D, Frazier KS, Welply A, et al. Transforming growth factor beta induces anchorage-independent growth of NRK fibroblasts via a connective tissue growth factor-dependent signaling pathway. Cell Growth Differ. 1997;8:61–68. [PubMed] [Google Scholar]
- 8.Kothapalli D, Hayashi N, Grotendorst GR. Inhibition of TGF-beta-stimulated CTGF gene expression and anchorage-independent growth by cAMP identifies a CTGF-dependent restriction point in the cell cycle. FASEB J. 1998;12:1151–1161. doi: 10.1096/fasebj.12.12.1151. [DOI] [PubMed] [Google Scholar]
- 9.Duncan MR, Frazier KS, Abramson S, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- 10.Derynck R, Feng XH. TGF-beta receptor signaling. Biochim Biophys Acta. 1997;1333:F105–F150. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 11.Roberts AB, Derynck R. Meeting report: signaling schemes for TGF-beta. Sci STKE. 2001;2001:pe43. doi: 10.1126/stke.2001.113.pe43. [DOI] [PubMed] [Google Scholar]
- 12.Na JI, Choi JW, Choi HR, et al. Rapid healing and reduced erythema after ablative fractional carbon dioxide laser resurfacing combined with the application of autologous platelet-rich plasma. Dermatol Surg. 2011;37:463–468. doi: 10.1111/j.1524-4725.2011.01916.x. [DOI] [PubMed] [Google Scholar]
- 13.Tan K, Duquette M, Liu JH, et al. Crystal structure of the TSP-1 type 1 repeats: a novel layered fold and its biological implication. J Cell Biol. 2002;159:373–382. doi: 10.1083/jcb.200206062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blake SM, Strasser V, Andrade N, et al. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J. 2008;27:3069–3080. doi: 10.1038/emboj.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrikopoulos P, Fraser SP, Patterson L, et al. Angiogenic functions of voltage-gated Na+ Channels in human endothelial cells: modulation of vascular endothelial growth factor (VEGF) signaling. J Biol Chem. 2011;286:16846–16860. doi: 10.1074/jbc.M110.187559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 17.Robson MC. The role of growth factors in the healing of chronic wounds. Wound Repair Regen. 1997;5:12–17. doi: 10.1046/j.1524-475X.1997.50106.x. [DOI] [PubMed] [Google Scholar]
- 18.Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–528. doi: 10.1016/s0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 19.Boateng JS, Matthews KH, Stevens HN, et al. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97:2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 20.Barrientos S, Brem H, Stojadinovic O, et al. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569–578. doi: 10.1111/wrr.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pakyari M, Farrokhi A, Maharlooei MK, et al. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care (New Rochelle) 2013;2:215–224. doi: 10.1089/wound.2012.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 23.Yuan LL, Adams JP, Swank M, et al. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinh NT, Prive A, Kheir L, et al. Involvement of KATP and KvLQT1 K+ channels in EGF-stimulated alveolar epithelial cell repair processes. Am J Physiol Lung Cell Mol Physiol. 2007;293:L870–L882. doi: 10.1152/ajplung.00362.2006. [DOI] [PubMed] [Google Scholar]
- 25.Veliceasa D, Ivanovic M, Hoepfner FT, et al. Transient potential receptor channel 4 controls thrombospondin-1 secretion and angiogenesis in renal cell carcinoma. FEBS J. 2007;274:6365–6377. doi: 10.1111/j.1742-4658.2007.06159.x. [DOI] [PubMed] [Google Scholar]