Fig. 12.
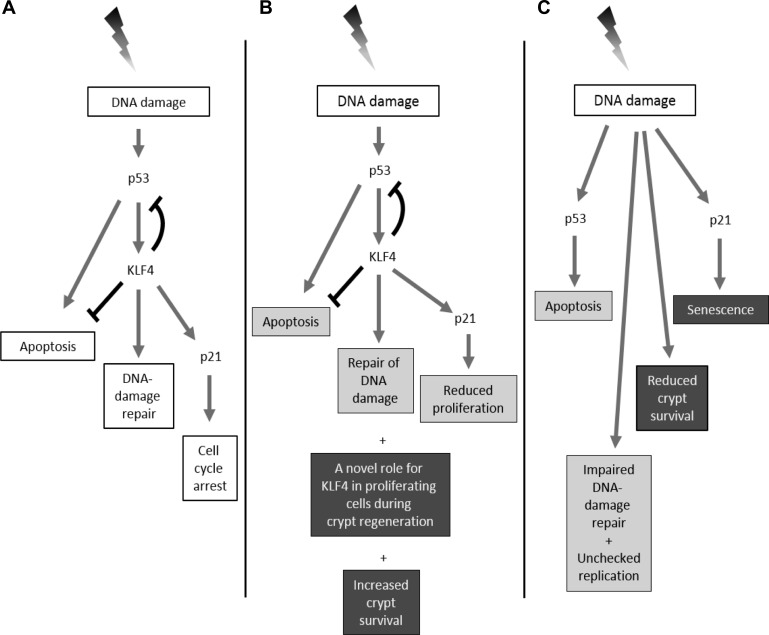
Model comparing the role of Klf4 in vitro (A) and in vivo (B and C) after γ-irradiation. A: in in vitro models, the expression of KLF4 is activated in a p53-dependent manner after γ-irradiation that then leads to increased p21 and decreased BAX expression. A net effect is to steer cells away from apoptosis and toward cell-cycle arrest. B: in vivo the interaction between p53, Klf4, and p21 might still hold in the intestinal epithelium following radiation injury; however, Klf4 might be required but not necessary for regulating the expression of p21. Klf4 is proposed to have short-term and a long-term roles in the intestinal epithelium following radiation injury. During the short term (gray boxes), Klf4 suppresses apoptosis and proliferation and is involved in DNA-repair response. During the long term (black boxes), Klf4 plays a role in increased crypt survival and might have a novel role in proliferation during the regenerative phase of the intestinal epithelium. C: in vivo deletion of Klf4 leads in the short term (gray boxes) to elevated p53, increased apoptosis, impaired DNA-damage repair, and unchecked proliferation. The expression of p21 shifts to a Klf4-independent pathway. In the long term (black boxes), p21 might divert crypt-cell fate toward senescence, and there is overall reduced crypt survival.