Figure 7.
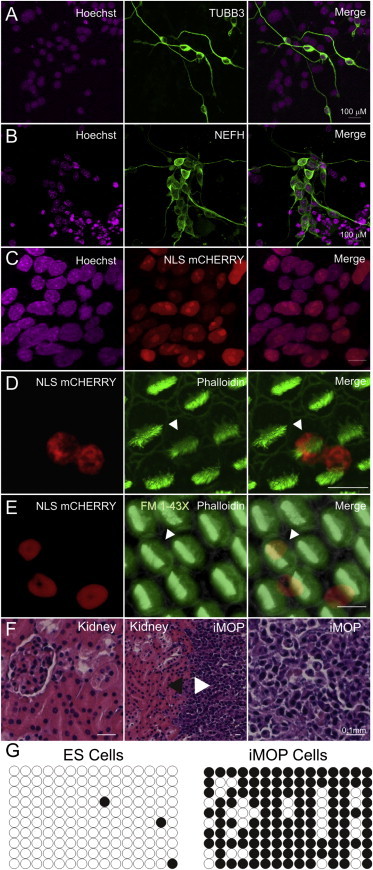
Differentiation of iMOP Cells in Different Cellular Contexts and Culture Conditions
(A) iMOP cells spontaneously differentiated into neurons and are marked by TUBB3 (neuronal β-III tubulin) using a Tuj1 antibody.
(B) iMOP-derived neurons are also marked by NEFH (neurofilament).
(C) Nuclei of progenitor cells, marked here by Hoechst staining, were genetically labeled to express nuclear mCherry fluorescent protein. Merged fluorescence shows robust colocalization.
(D) mCherry-labeled iMOP cells in chick otocysts form hair cells, as shown by phalloidin labeling of hair bundles (arrowhead), as well as supporting cells.
(E) A mouse iMOP-derived hair cell (marked by arrowhead) accumulated FM1-43X to a similar extent as endogenous chick hair cells.
(F) Hematoxylin and eosin staining of kidney. iMOP cells were injected into the kidney capsule and formed an encapsulated mass (white arrowhead) next to the kidney (black arrowhead). Undifferentiated iMOP cells make up the encapsulated mass. Scale bars represent 10 μm unless noted otherwise.
(G) Methylation status of the Oct4 promoter region in ESCs and iMOP cells. Each CpG dinucleotide pair along the Oct4 regulatory region is denoted by a circle. Filled and unfilled circles correspond to methylated and unmethylated basepairs, respectively. Ten sequences were analyzed for each cell line.
