Fig. 2.
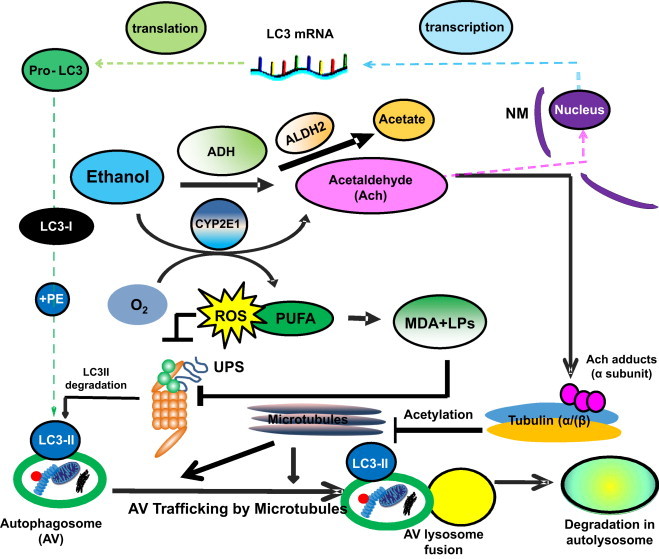
Multilevel regulation of autophagosome content by ethanol oxidation liver cells. During acute (or early) ethanol administration, metabolically-derived acetaldehyde (Ach) enhances AV formation in liver cells by increasing the level of LC3B mRNA, presumably by enhanced transcription. LC3B mRNA is then translated into pro-LC3, which matures to LC3-I. The latter is lipidated with phosphatidylethanolamine (PE) to form LC3-II, which attaches to the AV membrane. The AV is trafficked by microtubules to the lysosome for degradation. During habitual (chronic) ethanol exposure, CYP2E1 is induced, generating MDA and other lipid peroxides (LPs), which inhibit proteasome activity (depicted as UPS) and which also stabilizes LC3-II from degradation. Acetaldehyde (Ach) generated by ethanol oxidation forms adducts with proteins, including the α tubulin subunit. Acetaldehyde production is also closely associated with tubulin acetylation [100]. We hypothesize that formation of Ach-α-tubulin adducts or acetylated tubulin may block the intracellular polymerization of microtubules and prevent AV–lysosome fusion, thereby causing AV (LC3-II) and protein accumulation (proteopathy), which, with steatosis (fatty liver), contributes to alcohol-induced hepatomegaly. Reproduced and modified with permission from Ref. [44].
