Fig. 3.
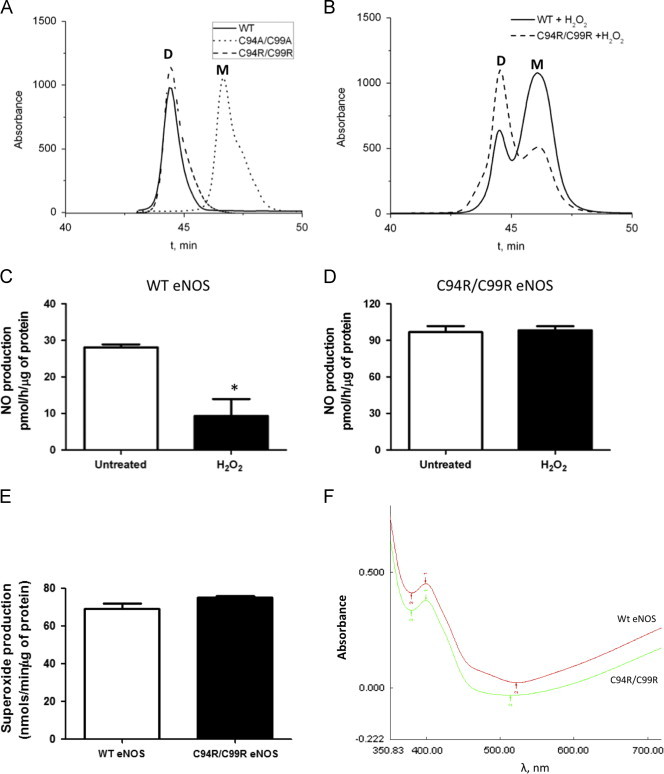
Characterization of C94R/C99R eNOS mutant. Gel filtration profiles (absorbance at λ260 nm) of WT (solid)-, C94R/C99R (dash)- and C94A/C99A (dot)-eNOS in the presence and absence of H2O2 (0.5 mM). WT- and C94R/C99R-eNOS form dimeric proteins in contrast to the C94A/C99A-eNOS mutant (A). H2O2 treatment disrupts the dimeric structure of WT eNOS, but the C94R/C99R mutant is resistant to monomerization (B). The disruption of the dimeric structure of WT eNOS correlates with a reduction in NO generation (C). NO generation is maintained in the C94A/C99A-eNOS mutant exposed to H2O2 (D). NO production in the C94R/C99R-eNOS under basal conditions is significantly higher that WT-eNOS (C and D) although superoxide generation is similar (E). Both WT- and C94R/C99R-eNOS enzymes exhibited high spin heme absorption peak at 396 nm that indicates catalytically active enzymes. (F). Data are mean±SEM, N=3, ⁎p<0.05 vs. untreated.
