Fig. 1.
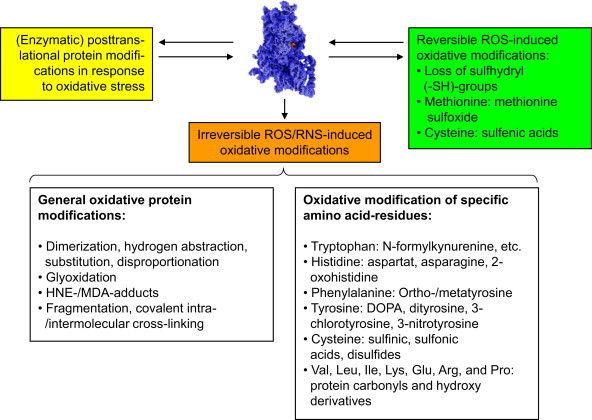
Typical ROS/RNS-mediated protein and side-chain modifications. This figure depicts some of the main reversible and irreversible protein modifications caused by ROS/RNS. The upper part shows different modifications some proteins can undergo in cells exposed to oxidative stress. Some of them are reversible oxidative modification (green box), that can be reversed by the cellular enzymatic machinery (see text below); another reversible pathway is the modification by cellular enzymes, that occurs in response to oxidative stress (yellow box). These modifications can be induced by ROS/RNS directly or by enzymatic reactions in response to ROS/RNS or a shifted redox-state of the cell, a common example for this is the so-called S-glutathionylation, mainly induced by oxidation of cysteine residues and reversed in an enzymatic way [23–25]. Another category is the formation of irreversible oxidative modifications by ROS/RNS that cannot be reversed by cellular enzymes (orange). Such proteins are usually recognized and degraded by specialized cellular enzymatic systems [8,9,26–30]. The lower part of the figure lists oxidative protein modification, classified by general principles or specific amino acid side-chains reactions. Reversible modifications are mainly found in cysteine and methionine residues, the only two amino acids that can be reduced/repaired by the cellular antioxidative enzymatic machinery. Methionine sulfoxide (MetSO) can be reduced by the methionine sulfoxide reductases Msr-A (specific for the S-stereoisomer) and Msr-B (specific for the R-stereoisomer of MetSO); both of them (Msr-A/B) use thioredoxin (Th-(SH)2) as reducing elements; after this, Th-(S-S) is reduced to Thr-(SH)2 again by the enzyme thioredoxin reductase in a NADPH-consuming way. The other amino acid residue very susceptible to ROS/RNS is cysteine. Its oxidation causes in proteins intra- or intermolecular cross-links (disulfides). Similar to MetSO, cysteine can be reduced by thioltransferases, that use either glutathione (GSH) or reduced thioredoxin (Th-(SH)2) in order to reduce a disulfide (–S–S–) into two separate –SH-groups (sulfhydryls). Of the different stages of cysteine oxidation, only the formation of the cysteinyl radical (protein-Cys-S•) and oxidation to sulfenic acid (protein-Cys-SOH) is reversible, while oxidation to sulfinic and sulfonic acid is irreversible, despite of a single known and highly specialized exception: sulfiredoxin is actually able to reduce sulfinic acid (protein-Cys-SO2H) in peroxiredoxins in an ATP-consuming reaction [31]. A loss of SH-groups may result in protein mis-/unfolding, inactivation (catalytic center), decreased antioxidative capacity, as well as the loss of specific functions. The variation of irreversible protein modifications outnumber the reversible ones by far and have in common that they cannot be repaired/reduced by the antioxidative machinery of the cell. Such general modifications (left description field of the lower part of this figure) can be induced by attacks of highly reactive radicals like hydroxyl, that are able to induce fragmentation of the protein, while attack on glycine seems to play a major role, as well as on proline, histidine and lysine [32]; furthermore, histidine is important in the formation of covalent cross-links [33]. Other events are de- and transamination (of glutamine and asparagine residues) that even can occur in a spontaneous way and does not have to be mediated/induced by ROS/RNS [34]. Furthermore, the formation of so-called advanced glycation end products (AGE's) [20,35,36] has been shown: Nε-carboxymethyllysine (CML) and Nε-carboxyethyllysine (CEL), as well as different glyoxal-lysine dimers (GOLDs) and methylglyoxal-lysine dimers (MOLDs) or pentosidine [37]. These AGEs are products of sugars and proteins, forming glycated proteins that may occur also from methylglyoxal, a potent glycating agent derived from trioses. Very prone to oxidative modifications are also the lipids in a cell. After ROS/RNS-mediated damage, amongst others highly reactive aldehydes are formed, that are able to react with proteins. The main reactive aldehydes are 4-hydroxy-2,3-nonenal (HNE, one of the most abundant products of lipid peroxidation, a bifunctional aldehyde, able to covalently cross-link proteins via reaction with either cysteine, lysine or histidine, followed by reaction with a lysine residue of another protein) [38], 4-hydroxyhexenal (HHE), malondialdehyde (MDA, forms Nε-malondialdehydelysine with lysine residues or the fluorescent adduct 1,4-dihydropyridine-3,5-dicarbaldehydes) [39]. The aldehydes glyoxal and acrolein react mainly with lysine, arginine, and histidine. The according end products of the mentioned reactions are referred to in the literature as “advanced lipid peroxidation end products” (ALEs)[37]. A typical step in the fragmentation of the protein backbone is the formation of an alkoxyl radical within the protein, that can decay either via the so-called diamide or α-amination pathways [40]. The irreversible oxidative modifications of specific residues show a large variety, but in biological systems several predominant modifications can be found some of them listed in the right description field of the lower part of this figure. In cells, the formation of 3-nitrotyrosine is mainly a hint to the presence of peroxynitrite (ONOO−), and thus the immunochemical detection of 3-nitrotyrosine became a quantitative and qualitative marker for ONOO−-mediated protein oxidation [41]. Dityrosines are mainly formed via the reaction of two tyrosyl radicals [42]. Those can be formed by the reaction of tyrosine side chains with hydroxyl radicals, hypochlorite or peroxynitrite [43]. Furthermore, hydroxyl radical mediated hydroxylation of phenylalanine, tyrosine and tryptophan plays a major role as well as comparable reactions of histidine, forming 2-oxohistidine [44]. Protein carbonyls [3,5] are the most abundant oxidative protein modification – their rate of formation is about 10-times higher than for any other oxidative protein modification. Protein carbonyls are mainly formed by oxidation of valine, leucine, isoleucine, lysine, glutamine, arginine, and proline side chains. Due to their high occurrence and establishment of easy to handle methods, protein carbonyls are the most often used quantitative marker of oxidative proteinmodification. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
