Abstract
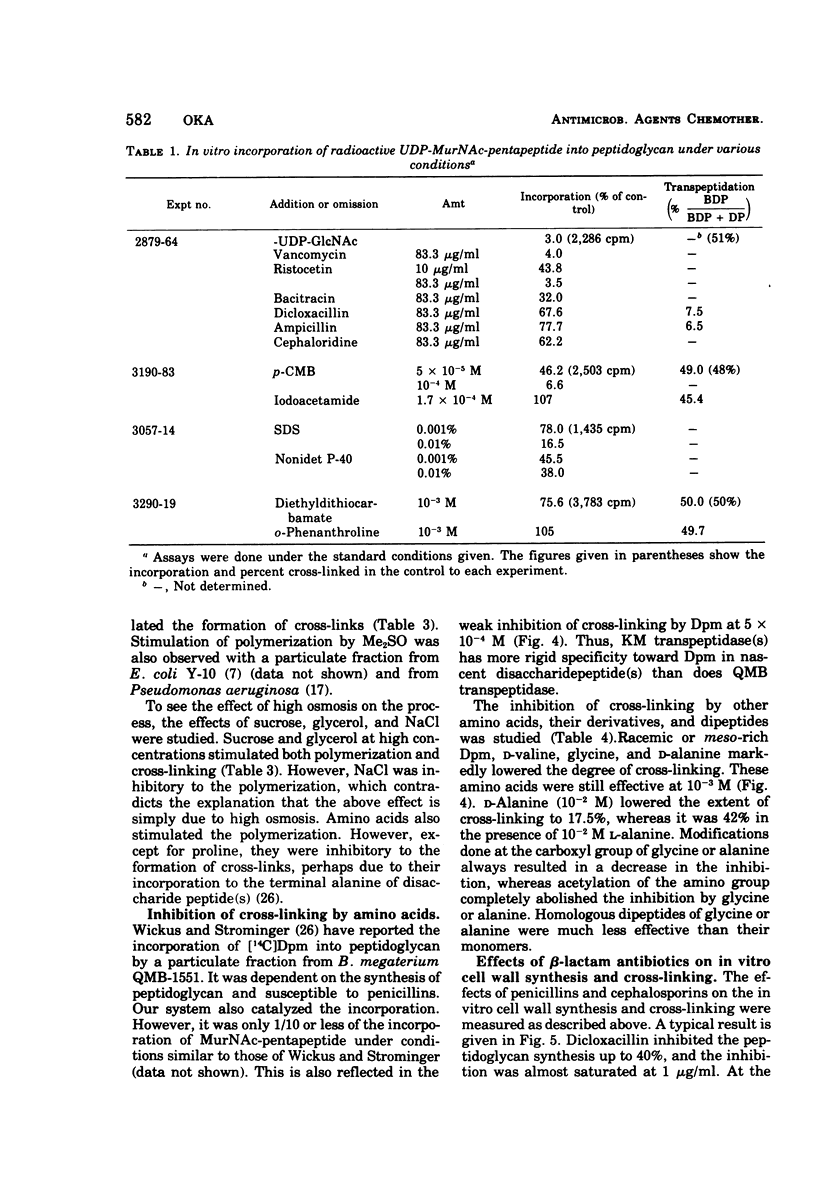
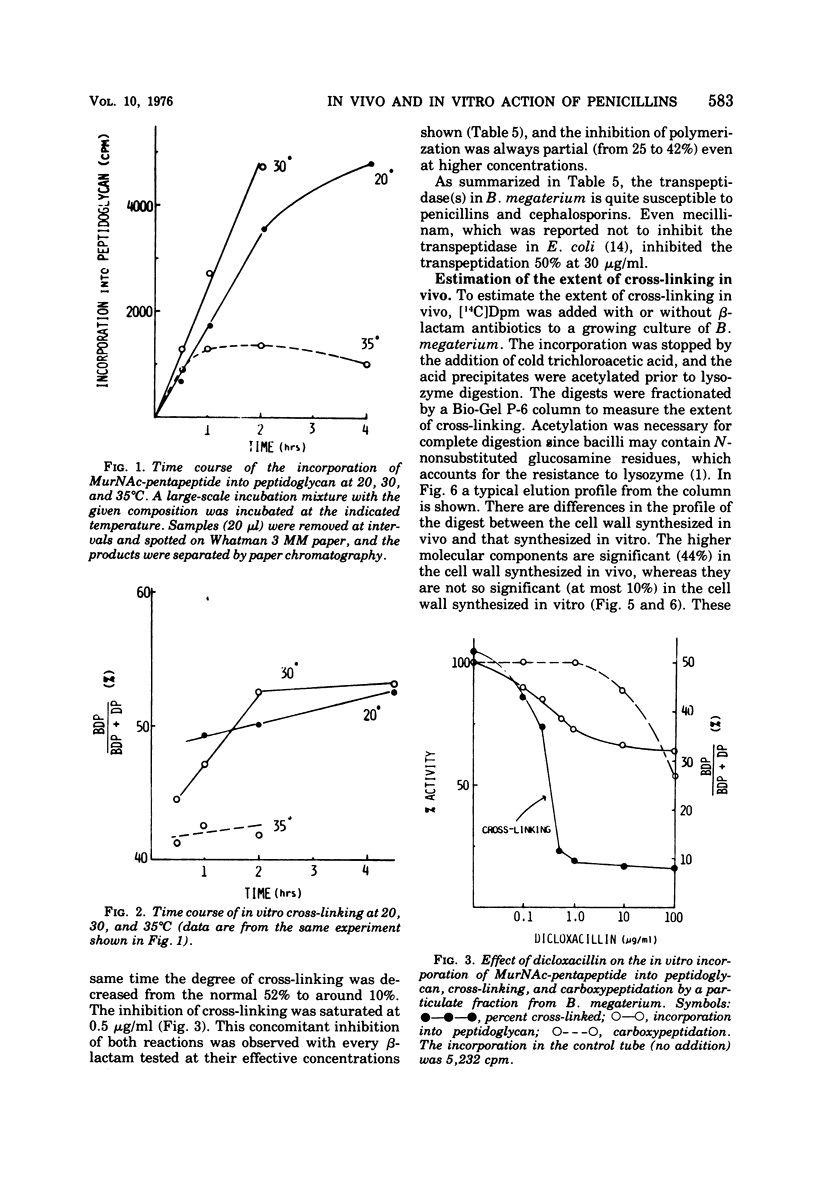
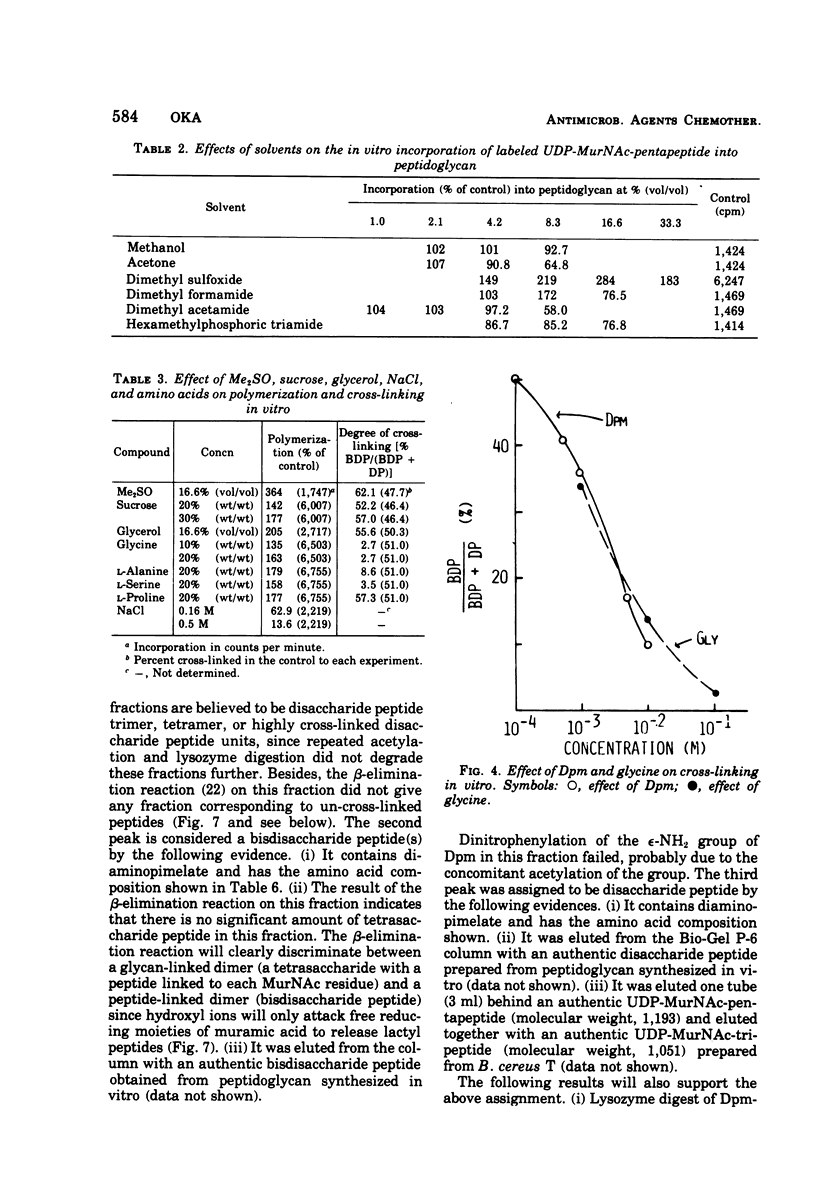
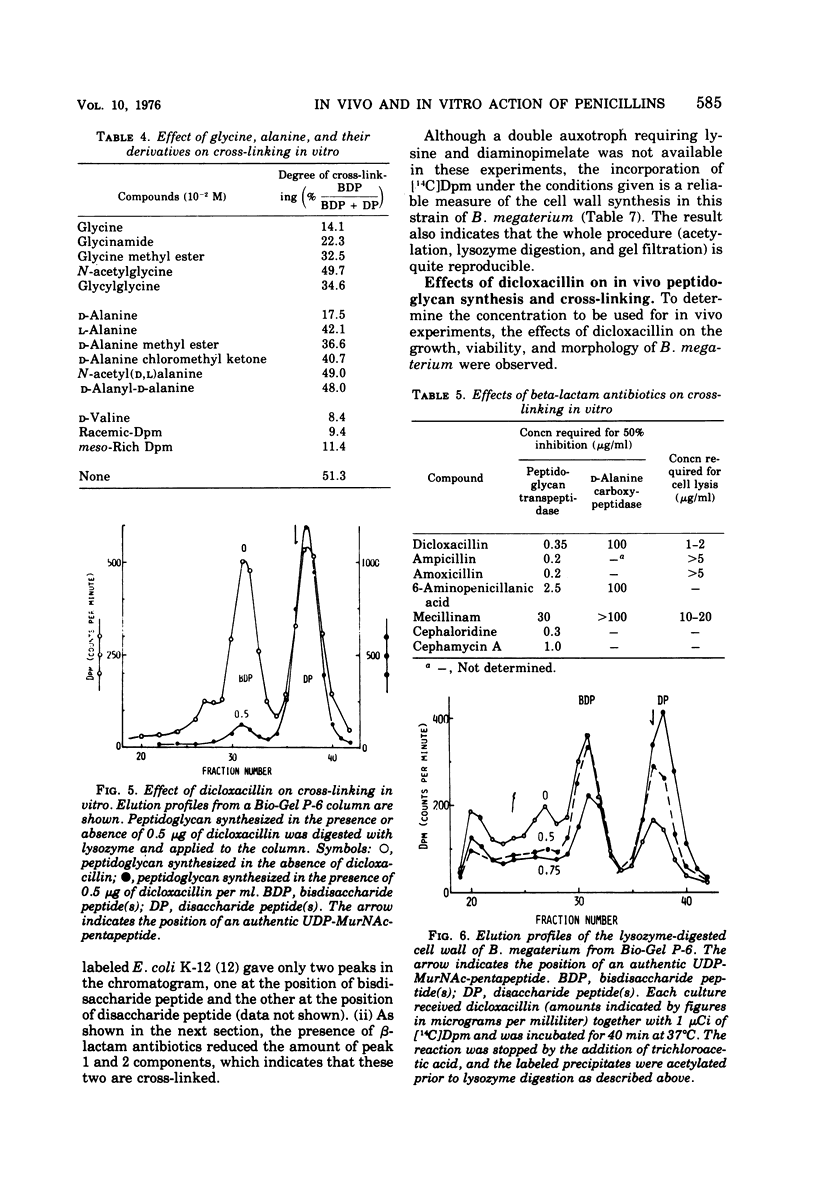
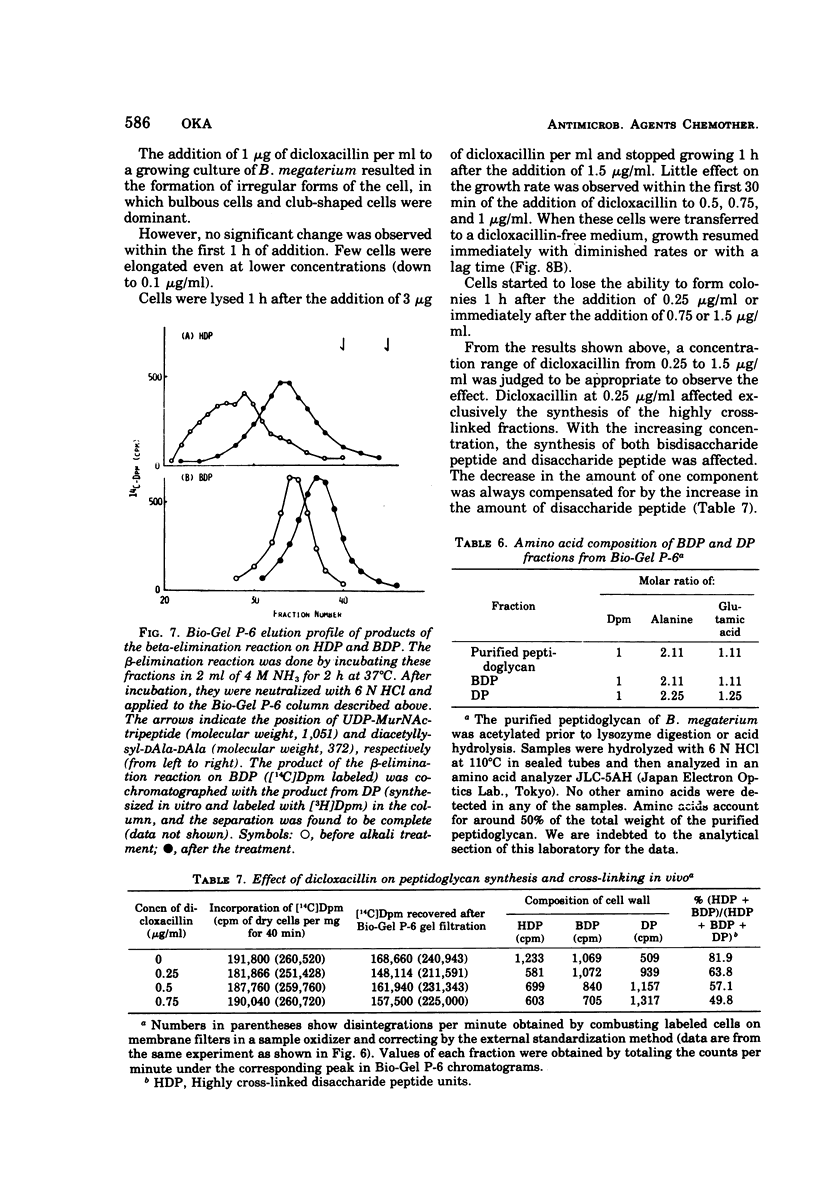
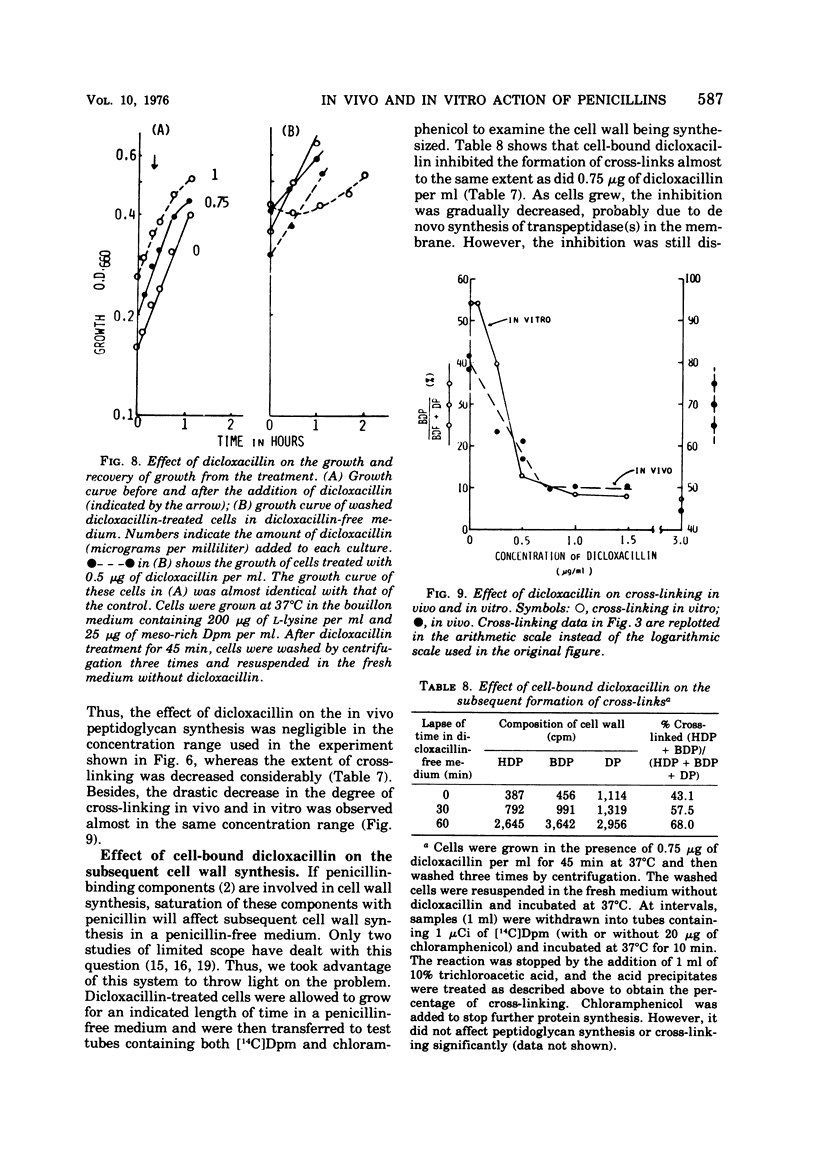
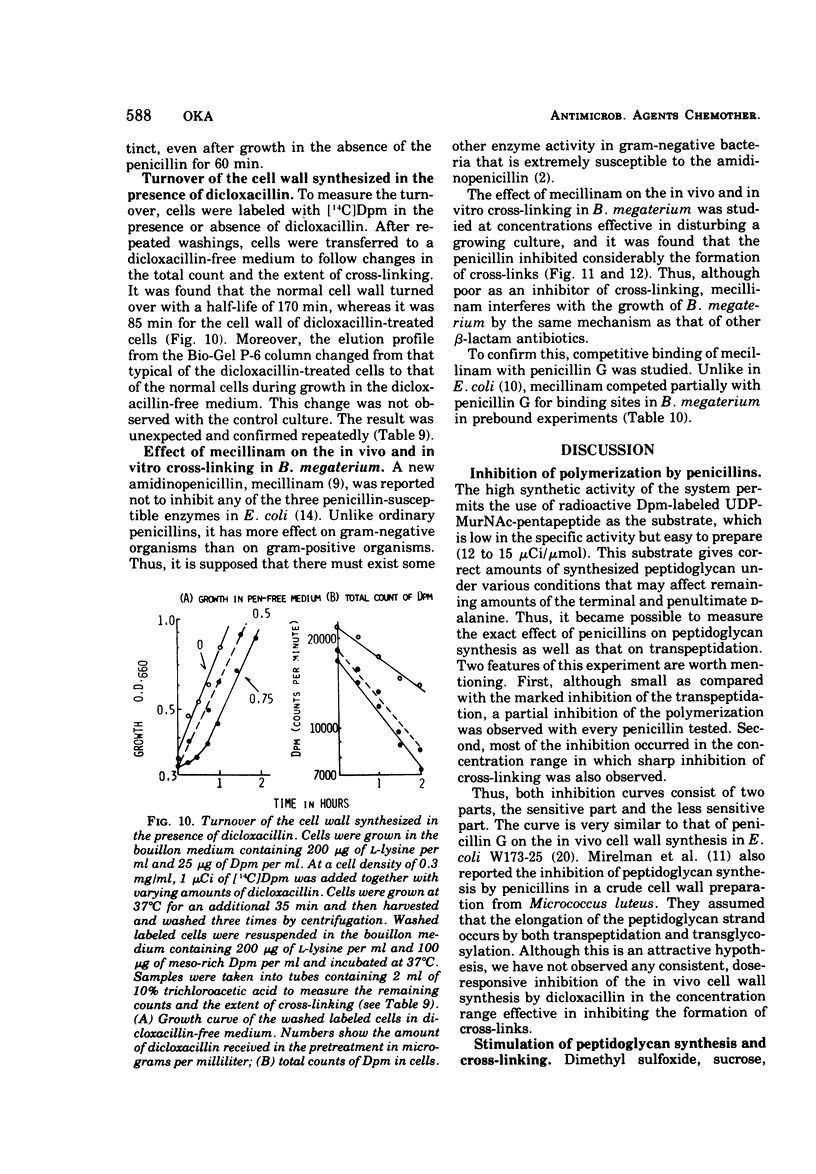
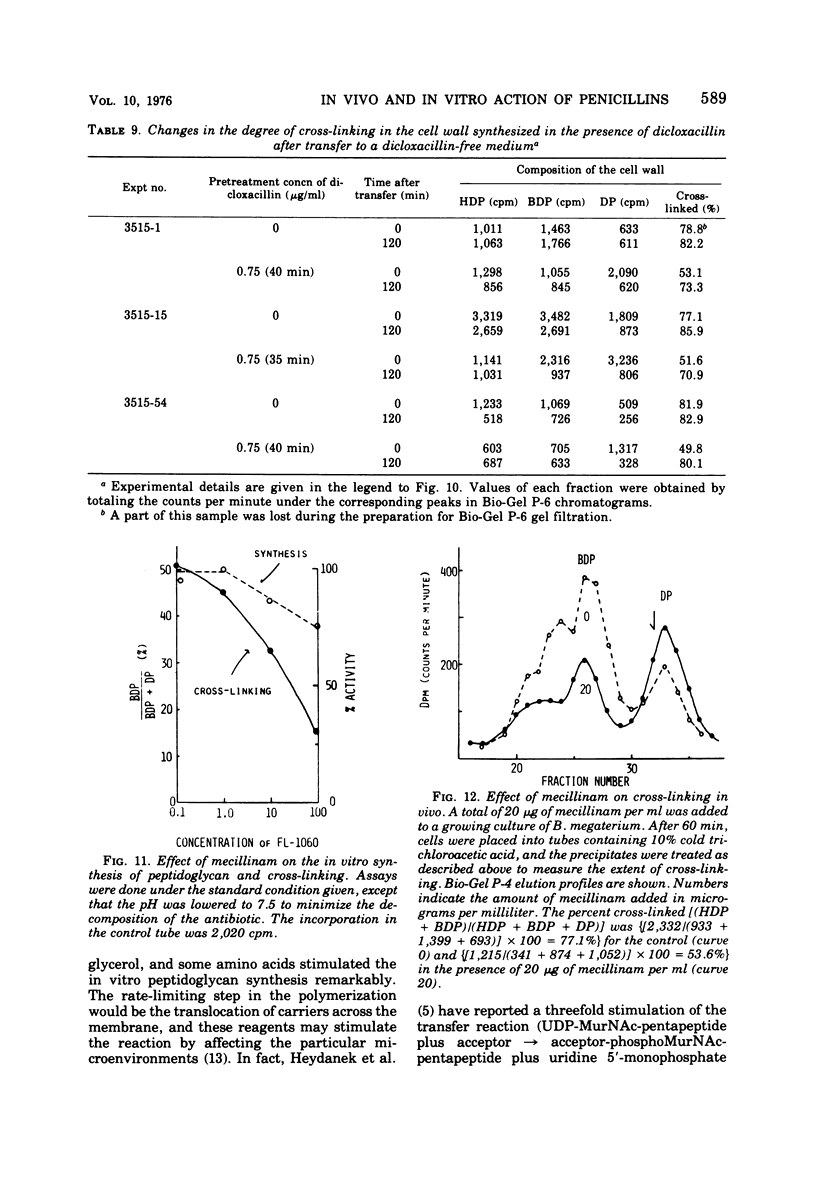
A new system in which the in vivo and in vitro formation of cross-links in the peptidoglycan of Bacillus megaterium can be compared directly has been developed. The method for the determination of the in vivo cross-linking consists of lysozyme digestion of acetylated [14C]diaminopimelic acid-labeled cells and Bio-Gel P-6 gel filtration of the digest. The elution profile indicates the cell wall synthesized in vivo consists of highly cross-linked fractions (44%), bisdisaccharide peptide(s) (38%), and disaccharide peptide(s) (18%). The in vitro system showed a high synthetic activity of cross-linked peptidoglycan. The synthesis was inhibited completely by 83.3 μg of ristocetin or vancomycin per ml or 10−4 M p-chloromercuribenzoate and inhibited only partially by penicillins. The polymerization was stimulated by high concentrations of sucrose, glycerol, amino acids, or dimethyl sulfoxide. The formation of cross-links was inhibited 50% at 0.3 μg of dicloxacillin per ml and 90% at 0.5 μg or more. It was also stimulated by high concentrations of sucrose, glycerol, or dimethyl sulfoxide. Effective concentrations of dicloxacillin on the growth, viability, and morphology of B. megaterium were determined. Sharp inhibition of cross-linking occurred in vivo and in vitro at these effective concentrations, whereas the incorporation of [14C]-diaminopimelate into bacterial cells was not affected at all. Cell-bound dicloxacillin reduced severely the degree of cross-linking in the cell wall synthesized after transfer to a dicloxacillin-free medium. Cell wall synthesized in the presence of dicloxacillin showed a higher rate of turnover than did the normal cell wall. Moreover, disaccharide peptide(s) was degraded faster than was bisdisaccharide peptide(s) in dicloxacillin-treated cells. From these observations, the primary target of penicillin action in B. megaterium is discussed in relation to the inhibition of cross-linking, penicillin-binding components, and cell lysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Nakatani T., Nakayama K., Ito E. Occurrence of N-nonsubstituted glucosamine residues in peptidoglycan of lysozyme-resistant cell walls from Bacillus cereus. J Biol Chem. 1972 Oct 10;247(19):6312–6322. [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordham W. D., Gilvarg C. Kinetics of cross-linking of peptidoglycan in Bacillus megaterium. J Biol Chem. 1974 Apr 25;249(8):2478–2482. [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Perkins H. R., Nieto M. Kinetics of concomitant transfer and hydrolysis reactions catalysed by the exocellular DD-carboxypeptidase-transpeptidase of streptomyces R61. Biochem J. 1973 Nov;135(3):483–492. doi: 10.1042/bj1350483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydanek M. G., Jr, Struve W. G., Neuhaus F. C. On the initial stage in peptidoglycan synthesis. 3. Kinetics and uncoupling of phospho-N-acetylmuramyl-pentapeptide translocase (uridine 5'-phosphate). Biochemistry. 1969 Mar;8(3):1214–1221. doi: 10.1021/bi00831a056. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lund F., Tybring L. 6 -amidinopenicillanic acids--a new group of antibiotics. Nat New Biol. 1972 Apr 5;236(66):135–137. doi: 10.1038/newbio236135a0. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S., Kamiryo T., Blumberg P. M., Linnett P., Willoughby E., Strominger J. L. Mechanism of action and development of resistance to a new amidino penicillin. J Bacteriol. 1974 Feb;117(2):578–587. doi: 10.1128/jb.117.2.578-587.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Studies on the elongation of bacterial cell wall peptidoglycan and its inhibition by penicillin. Ann N Y Acad Sci. 1974 May 10;235(0):326–347. doi: 10.1111/j.1749-6632.1974.tb43275.x. [DOI] [PubMed] [Google Scholar]
- Nagai K., Tamura G. Mutant of Escherichia coli with thermosensitive protein in the process of cellular division. J Bacteriol. 1972 Nov;112(2):959–966. doi: 10.1128/jb.112.2.959-966.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. T., Burman L. FL-1060: a new penicillin with a unique mode of action. Biochem Biophys Res Commun. 1973 Apr 16;51(4):863–868. doi: 10.1016/0006-291x(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Park J. T., Edwards J. R., Wise E. M., Jr In vivo studies on the uptake and binding of beta-lactam antibiotics in relation to inhibition of wall synthesis and cell death. Ann N Y Acad Sci. 1974 May 10;235(0):300–309. doi: 10.1111/j.1749-6632.1974.tb43273.x. [DOI] [PubMed] [Google Scholar]
- Park J. T., Griffith M. E., Stevenson I. Resistance to penicillin in mutants of a penicillinase-negative organism, Staphylococcus aureus H. J Bacteriol. 1971 Dec;108(3):1154–1160. doi: 10.1128/jb.108.3.1154-1160.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presslitz J. E., Ray V. A. DD-carboxypeptidase and peptidoglycan transpeptidase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1975 May;7(5):578–581. doi: 10.1128/aac.7.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E. Peptidoglycan synthesis in bacilli. I. Effect of temperature on the in vitro system from Bacillus megaterium and Bacillus stearothermophilus. Biochim Biophys Acta. 1971 May 18;237(2):239–254. [PubMed] [Google Scholar]
- Rogers H. J. The inhibition of mucopeptide synthesis by benzylpenicillin in relation to irreversible fixation of the antibiotic by staphylococci. Biochem J. 1967 Apr;103(1):90–102. doi: 10.1042/bj1030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickus G. G., Strominger J. L. Penicillin-sensitive transpeptidation during peptidoglycan biosynthesis in cell-free preparations from Bacillus megaterium. II. Effect of penicillins and cephalosporins on bacterial growth and in vitro transpeptidation. J Biol Chem. 1972 Sep 10;247(17):5307–5311. [PubMed] [Google Scholar]



